Professor Doudna was awarded the 2020 Nobel Prize in Chemistry with Professor Emmanuelle Charpentier for their pioneering work in CRISPR genome editing. The first genome editing therapy (Casgevy) was just FDA approved, only a decade after the CRISPR-Cas9 editing system discovery. But It’s just the beginning of a much bigger impact story for medicine and life science.
Ground Truths podcasts are now on Apple and Spotify.
And if you prefer videos, they are posted on YouTube
Transcript with links to audio and relevant external links
Eric Topol (00:06):
This is Eric Topol with Ground Truths, and I'm really excited today to have with me Professor Jennifer Doudna, who heads up the Innovative Genomics Institute (IGI) at UC Berkeley, along with other academic appointments, and as everybody knows, was the Nobel laureate for her extraordinary discovery efforts with CRISPR genome editing. So welcome, Jennifer.
Jennifer Doudna (00:31):
Hello, Eric. Great to be here.
Eric Topol (00:34):
Well, you know we hadn't met before, but I felt like I know you so well because this is one of my favorite books, The Code Breaker. And Walter Isaacson did such a wonderful job to tell your story. What did you think of the book?
My interview with Walter Isaacson on The Code Breaker, a book I highly recommend
Jennifer Doudna (00:48):
I thought Walter did a great job. He's a good storyteller, and as you know from probably from reading it or maybe talking to others about it, he wrote a page turner. He actually really dug into the science and all the different aspects of it that I think created a great tale.
Eric Topol (01:07):
Yeah, I recommended highly. It was my favorite book when it came out a couple years ago, and it is a page turner. In fact, I just want to read one, there's so many quotes out of it, but in the early part of the book, he says, “the invention of CRISPR and the plague of Covid will hasten our transition to the third great revolution of modern times. These revolutions arose from the discovery beginning just over a century ago, of the three fundamental kernels of our existence, the atom, the bit, and the gene.” That kind of tells a big story just in one sentence, but I thought I’d start with the IGI, the institute that you have set up at Berkeley and what its overall goals are.
Jennifer Doudna (01:58):
Right. Well, let's just go back a few years maybe to the origins of this institute and my thinking around it, because in the early days of CRISPR, it was clear that we were really at a moment that was quite unique in the sense that there was a transformative technology. It was going to intersect with lots of other discoveries and technologies. And I work at a public institution and my question to myself was, how can I make sure that this powerful tool is first of all used responsibly and secondly, that it's used in a way that benefits as many people as possible, and it's a tall order, but clearly we needed to have some kind of a structure that would allow people to work together towards those goals. And that was really the mission behind the IGI, which was started as a partnership between UC Berkeley and UCSF and now actually includes UC Davis as well.
The First FDA Approved Genome Editing
Eric Topol (02:57):
I didn't realize that. That's terrific. Well, this is a pretty big time because 10 years or so, I guess starting to be 11 when you got this thing going, now we're starting to see, well, hundreds of patients have been treated and in December the FDA approved the first CRISPR therapy for sickle cell disease, Casgevy. Is that the way you say it?
Jennifer Doudna (03:23):
Casgevy, yeah.
Eric Topol (03:24):
That must have felt pretty good to see if you go from the molecules to the bench all the way now to actually treating diseases and getting approval, which is no easy task.
Jennifer Doudna (03:39):
Well, Eric, for me, I'm a biochemist and somebody who has always worked on the fundamentals of biology, and so it's really been extraordinary to see the pace at which the CRISPR technology has been adopted, and not just for fundamental research, but also for real applications. And Casgevy is sort of the crowning example of that so far, is that it's really a technology that we can already see how it's being used to, I think it's fair to say, effectively cure a genetic disease for the first time. Really amazing.
Genome Editing is Not the Same as Gene Therapy
Eric Topol (04:17):
Yeah. Now I want to get back to that. I know there's going to be refinements about that. And of course, there's beta thalassemia, so we've got two already, and our mutual friend Fyodor Urnov would say two down 5,000 to go. But I think before I get to the actual repair of the sickle cell defect molecular defect, I think one of the questions I think that people listeners may not know is the differentiation of genome editing with gene therapy. I mean, as you know, there was recently a gene therapy approval for something like $4.25 million for metachromatic leukodystrophy. So maybe you could give us kind of skinny on how these two fundamental therapies are different.
Jennifer Doudna (05:07):
Right. Well, it's a great question because the terminology sounds kind of the same, and so it could be confusing. Gene therapy goes back decades, I can remember gene therapy being discussed as an exciting new at the time, direction back when I was a graduate student. That was little while ago. And it refers to the idea that we can use a genetic approach for disease treatment or even for a cure. However, it fundamentally requires some mechanism of integrating new information into a genome. And traditionally that's been done using viruses, which are great at doing that. It's just that they do it wherever they want to do it, not necessarily where we want that information to go. And this is where CRISPR comes in. It's a technology allows precision in that kind of genetic manipulation. So it allows the scientist or the clinician to decide where to make a genetic change. And that gives us tremendous opportunity to do things with a kind of accuracy that hasn't been possible before.
Eric Topol (06:12):
Yeah, no question. That's just a footnote. My thesis in college at University of Virginia, 1975, I'm an old dog, was prospects for gene therapy in man. So it took a while, didn't it? But it's a lot better now with what you've been working on, you and your colleagues now and for the last decade for sure. Now, what I was really surprised about is it's not just of course, these hemoglobin disorders, but now already in phase two trials, you've got hereditary angioedema, which is a life-threatening condition, amyloidosis, cancer ex vivo, and also chronic urinary tract infections. And of course, there's six more others like autoimmune diseases like lupus and type 1 diabetes. So this is really blossoming. It's really extraordinary.
Eric Topol (07:11):
I mean, wow. So one of the questions I had about phages, because this is kind of going back to this original work and discovery, antimicrobial resistance is really a big problem and it's a global health crisis, and there's only two routes there coming up with new drugs, which has been slow and not really supported by the life science industry. And the other promising area is with phages. And I wonder, since this is an area you know so well, why haven't we put more, we're starting to see more trials in phages. Why haven't we doubled down or tripled down on this to help the antimicrobial resistance problem?
Jennifer Doudna (08:00):
Well, it's a really interesting area, and as you said, it's kind of one of those areas of science where I think there was interest a while ago and some effort was made for reasons that are not entirely clear to me, at least it fizzled out as a real focused field for a long time. But then more recently, people have realized that there's an opportunity here to take advantage of some natural biology in which viruses can infect and destroy microbes. Why aren't we taking better advantage of that for our own health purposes? So I personally am very excited about this area. I think there's a lot of fundamental work still to be done, but I think there's a tremendous opportunity there as well.
CRISPR 2.0
Eric Topol (08:48):
Yeah, I sure think we need to invest in that. Now, getting back to this sickle cell story, which is so extraordinary. This is kind of a workaround plan of getting fetal hemoglobin built up, but what about actually repairing, getting to fixing the lesion, if you will?
Eric Topol (09:11):
Yeah. Is that needed?
Jennifer Doudna (09:13):
Well, maybe it's worth saying a little bit about how Casgevy works, and you alluded to this. It's not a direct cure. It's a mechanism that allows activation of a second protein called fetal hemoglobin that can suppress the effect of the sickle cell mutation. And it's great, and I think for patients, it offers a really interesting opportunity with their disease that hasn't been available in the past, but at the same time, it's not a true cure. And so the question is could we use a CRISPR type technology to actually make a correction to the genetic defect that directly causes the disease? And I think the answer is yes. The field isn't there quite yet. It's still relatively difficult to control the exact way that DNA editing is occurring, especially if we're doing it in vivo in the body. But boy, many people are working on this, as you probably know. And I really think that's on the horizon.
Eric Topol (10:19):
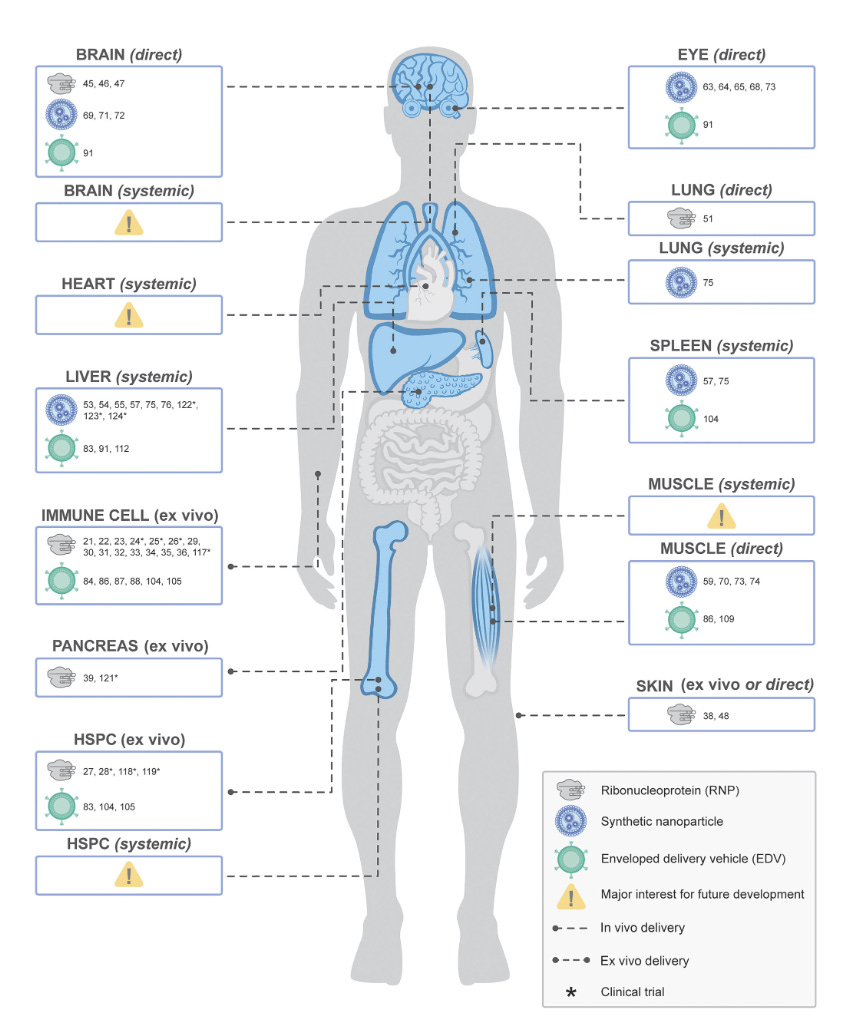
Yeah. Well, I think we want to get into the in vivo story as well because that, I think right now it's so complicated for a person to have to go through the procedure to get ultimately this treatment currently for sickle cell, whereas if you could do this in vivo and you could actually get the cure, that would be of the objective. Now, you published just earlier this month in PNAS a wonderful paper about the EDVs and the lipid nanoparticles that are ways that we could get to a better precision editing. These EDVs I guess if I have it right, enveloped virus-like particles. It could be different types, it could be extracellular vesicles or whatnot. But do you think that's going to be important? Because right now we're limited for delivery, we're limited to achieve the right kind of editing to do this highly precise. Is that a big step for the future?
Jennifer Doudna (11:27):
Really big. I think that's gating at the moment. Right now, as you mentioned, somebody that might want to get the drug Casgevy for sickle cell disease or thalassemia, they have to go through a bone marrow transplant to get it. And that means that it's very expensive. It's time consuming. It's obviously not pleasant to have to go through that. And so that automatically means that right now that therapy is quite restricted in the patients that it can benefit. But we imagine a day when you could get this type of therapy into the body with a one-time injection. Maybe someday it's a pill that could be taken where the gene editors target the right cells in the body. In diseases like that, it would be the stem cells in the bone marrow and carry out gene editing that can have a therapeutic benefit. And again, it's one of those ideas that sounds like science fiction, and yet already there's tremendous advance in that direction. And I think over the next, I don't know, I'm guessing 5 to 10 years we're going to see that coming online.
Editing RNA, the Epigenome, and the Microbiome
Eric Topol (12:35):
Yeah, I'm guessing just because there's so much work on the lipid nanoparticles to tweak them. And there's four different components that could easily be made so much better. And then all these virus-like proteins, I mean, it may happen even sooner. And it's really exciting. And I love that diagram in that paper. You have basically every organ of the body that isn't accessible now, potentially that would become accessible. And that's exciting because whatever blossoming we're seeing right now with these phase two trials ongoing, then you basically have no limits. And that I think is really important. So in vivo editing big. Now, the other thing that's cropped up in recent times is we've just been focused on DNA, but now there's RNA editing, there's epigenetic or epigenomic editing. What are your thoughts about that?
Jennifer Doudna (13:26):
Very exciting as well. It's kind of a parallel strategy. The idea there would be to, rather than making a permanent change in the DNA of a cell, you could change just the genetic output of the cell and or even make a change to DNA that would alter its ability to be expressed and to produce proteins in the cell. So these are strategies that are accessible, again, using CRISPR tools. And the question is now how to use them in ways that will be therapeutically beneficial. Again, topics that are under very active investigation in both academic labs and at companies.
Eric Topol (14:13):
Yeah. Now speaking of that, this whole idea of rejuvenation, this is Altos. You may I'm sure know my friend here, Juan Carlos Belmonte, who's been pushing on this for some time at Altos now formerly at Salk. And I know you helped advise Altos, but this idea of basically epigenetic, well using the four Yamanaka factors and basically getting cells that go to a state that are rejuvenated and all these animal models that show that it really happens, are you thinking that really could become a therapy in the times ahead in patients for aging or particular ideas that you have of how to use that?
Jennifer Doudna (15:02):
Well, you mentioned the company Altos. I mean, Altos and a number of other groups are actively investigating this. Not I would say specifically regarding genome editing, although being able to monitor and probably change gene functions that might affect the aging process could be attractive in the future. I think the hard question there is which genes do we tweak and how do we make sure that it's safe? And better than me I mean, that's a very difficult thing to study clinically because it takes time for one thing, and we probably don't have the best models either. So I think there are challenges there for sure. But along the way, I feel very excited about the kind of fundamental knowledge that will come from those studies. And in particular, this question of how tissues rejuvenate I think is absolutely fascinating. And some organisms do this better than others. And so, understanding how that works in organisms that are able to say regrow a limb, I think can be very interesting.
Eric Topol (16:10):
And that gets me to that recent study. Well, as you well know, there's a company Verve that's working on the familial hypercholesterolemia and using editing with the PCSK9 through the liver and having some initial, at least a dozen patients have been treated. But then this epigenetic study of editing in mice for PCSK9 also showed results. Of course, that's much further behind actually treating patients with base editing. But it's really intriguing that you can do some of these things without having to go through DNA isn't it?
Jennifer Doudna (16:51):
Amazing, right? Yeah, it's very interesting.
Reducing the Cost of Genome Editing
Eric Topol (16:54):
Wild. Now, one of the things of course that people bring up is, well, this is so darn expensive and it's great. It's a science triumph, but then who can get these treatments? And recently in January, you announced a Danaher-IGI Beacon, and maybe you can tell us a bit about that, because again, here's a chance to really markedly reduce the cost, right?
Jennifer Doudna (17:25):
That's right. That's the vision there. And huge kudos to my colleague Fyodor Urnov, who really spearheaded that effort and leads the team on the IGI side. But the vision there was to partner with a company that has the ability to manufacture molecules in ways that are very, very hard, of course, for academic labs and even for most companies to do. And so the idea was to bring together the best of genome editing technology, the best of clinical medicine, especially focused on rare human diseases. And this is with our partners at UCSF and with the folks in the Danaher team who are experts at downstream issues of manufacturing. And so the hope there is that we can bring those pieces together to create ways of using CRISPR that will be cost effective for patients. And frankly, we'll also create a kind of roadmap for how to do this, how to do this more efficiently. And we're kind of building the plane while we're flying it, if you know what I mean. But we're trying to really work creatively with organizations like the FDA to come up with strategies for clinical trials that will maintain safety, but also speed up the timeline.
Eric Topol (18:44):
And I think it's really exciting. We need that and I'm on the scientific advisory board of Danaher, a new commitment for me. And when Fyodor presented that recently, I said, wow, this is exciting. We haven't really had a path to how to get these therapies down to a much lower cost. Now, another thing that's exciting that you're involved in, which I think crosses the whole genome editing, the two most important things that I've seen in my lifetime are genome editing and AI, and they also work together. So maybe before we get into AI for drug discovery, how does AI come into play when you're thinking about doing genome editing?
Jennifer Doudna (19:34):
Well, the thing about CRISPR is that as a tool, it's powerful not only as a one and done kind of an approach, but it's also very powerful genomically, meaning that you can make large libraries of these guide RNAs that allow interrogation of many genes at once. And so that's great on the one hand, but it's also daunting because it generates large collections of data that are difficult to manually inspect. And in some cases, I believe really very, very difficult to analyze in traditional ways. But imagine that we have ways of training models that can look at genetic intersections, ways that genes might be affecting the behavior of not only other genes, but also how a person responds to drugs, how a person responds to their environment and allows us to make predictions about genetic outcomes based on that information. I think that's extremely exciting, and I definitely think that over the next few years we'll see that kind of analysis coming online more and more.
Eric Topol (20:45):
Yeah, the convergence, I think is going to be, it's already being done now, but it's just going to keep building. Now, Demis Hassabis, who one of the brilliant people in the field of AI leads the whole Google Deep Mind AI efforts now, but he formed after AlphaFold2 behaving to predict proteins, 200 million proteins of the universe. He started a company Isomorphic Labs as a way to accelerate using AI drug discovery. What can you tell us about that?
Jennifer Doudna (21:23):
It's exciting, isn't it? I'm on the SAB for that company, and I think it's very interesting to see their approach to drug discovery. It's different from what I've been familiar with at other companies because they're really taking a computational lens to this challenge. The idea there is can we actually predict things like the way a small molecule might interact with a particular protein or even how it might interact with a large protein complex. And increasingly because of AlphaFold and programs like that, that allow accurate prediction of structures, it's possible to do that kind of work extremely quickly. A lot of it can be done in silico rather than in the laboratory. And when you do get around to doing experiments in the lab, you can get away with many fewer experiments because you know the right ones to do. Now, will this actually accelerate the rate at which we get to approved therapeutics? I wonder about your opinion about that. I remain unsure.
Editing Out Alzheimer’s Risk Alleles
Eric Topol (22:32):
Yeah. I mean, we have one great success story so far during the pandemic Baricitinib, a drug that repurposed here, a drug that was for rheumatoid arthritis, found by data mining that have a high prospects for Covid and now saves lives in Covid. So at least that's one down, but we got a lot more here too. But it, it's great that Demis recruited you on the SAB for Isomorphic because it brings in a great mind in a different field. And it goes back to one of the things you mentioned earlier is how can we get some of this genome editing into a pill someday? Wow. Now, one of the things that for personal interest, as an APOE4 carrier, I'm looking to you to fix my APOE4 and give me APOE2. How can I expect to get that done in the near future?
Jennifer Doudna (23:30):
Oh boy. Okay, we'll have to roll up our sleeves on that one. But it is appealing, isn't it? I think about it too. It's a fascinating idea. Could we get to a point someday where we can use genome editing as a prophylactic, not as a treatment after the fact, but as a way to actually protect ourselves from disease? And the APOE4 example is a really interesting one because there's really good evidence that by changing the type of allele that one has for the APOE gene, you can actually affect a person's likelihood of developing Alzheimer's in later life. But how do we get there? I think one thing to point out is that right now doing genome editing in the brain is, well, it's hard. I mean, it's very hard.
Eric Topol (24:18):
It a little bit's been done in cerebral spinal fluid to show that you can get the APOE2 switch. But I don't know that I want to sign up for an LP to have that done.
Jennifer Doudna (24:30):
Not quite yet.
Eric Topol (24:31):
But someday it's wild. It's totally wild. And that actually gets me back to that program for coronary heart disease and heart attacks, because when you're treating people with familial hypercholesterolemia, this extreme phenotype. Someday and this goes for many of these rare diseases that you and others are working on, it can have much broader applicability if you have a one-off treatment to prevent coronary disease and heart attacks and you might use that for people well beyond those who have an LDL cholesterol that are in the thousands. So that's what I think a lot of people don't realize that this editing potential isn't just for these monogenic and rare diseases. So we just wanted to emphasize that. Well, this has been a kind of wild ride through so much going on in this field. I mean, it is extraordinary. What am I missing that you're excited about?
Jennifer Doudna (25:32):
Well, we didn't talk about the microbiome. I'll just very briefly mention that one of our latest initiatives at the IGI is editing the microbiome. And you probably know there are more and more connections that are being made between our microbiome and all kinds of health and disease states. So we think that being able to manipulate the microbiome precisely is going to open up another whole opportunity to impact our health.
Can Editing Slow the Aging Process?
Eric Topol (26:03):
Yeah, I should have realized that when I only mentioned two layers of biology, there's another one that's active. Extraordinary, just going back to aging for a second today, there was a really interesting paper from Irv Weissman Stanford, who I'm sure you know and colleagues, where they basically depleted the myeloid stem cells in aged mice. And they rejuvenated the immune system. I mean, it really brought it back to life as a young malice. Now, there probably are ways to do that with editing without having to deplete stem cells. And the thought about other ways to approach the aging process now that we're learning so much about science and about the immune system, which is one of the most complex ones to work in. Do you have ideas about that are already out there that we could influence the aging process, especially for those of us who are getting old?
Jennifer Doudna (27:07):
We're all on that path, Eric. Well, I guess the way that I think about it is I like to think that genome editing is going to pave the way to make those kinds of fundamental discoveries. I still feel that there's a lot of our genetics that we don't understand. And so, by being able to manipulate genes precisely and increasingly to look at how genes interact with each other, I think one fundamental question it relates to aging actually is why do some of us age at a seemingly faster pace than others? And it must have to do at least in part with our genetic makeup and how we respond to our environment. So I definitely think there are big opportunities there, really in fundamental research initially, but maybe later to actually change those kinds of things.
Eric Topol (28:03):
Yeah, I'm very impressed in recent times how much the advances are being made at basic science level and experimental models. A lot of promise there. Now, is there anything about this field that you worry about that keeps you up at night that you think, besides, we talked about that we got to get the cost down, we have to bridge health inequities for sure, but is there anything else that you're concerned about right now?
Jennifer Doudna (28:33):
Well, I think anytime a new technology goes into clinical trials, you worry that things may get out ahead of their skis, and there may be some overreach that happens. I think we haven't really seen that so far in the CRISPR field, which is great. But I guess I remain cautious. I think that we all saw what happened in the field of gene therapy now decades ago, but that really put a poll on that field for a long time. And so, I definitely think that we need to continue to be very cautious as gene editing continues to advance.
Eric Topol (29:10):
Yeah, no question. I think the momentum now is getting past that point where you would be concerned about known unknowns, if you will, things that going back to the days of the Gelsinger crisis. But it's really extraordinary. I am so thrilled to have this conversation with you and to get a chance to review where the field is and where it's going. I mean, it's exploding with promise and potential well beyond and faster. I mean, it takes a drug 17 years, and you've already gotten this into two treatments. I mean, I'm struck when you were working on this, how you could have thought that within a 10-year time span you'd already have FDA approvals. It's extraordinary.
Jennifer Doudna (30:09):
Yeah, we hardly dared hope. Of course, we're all thrilled that it went that fast, but I think it would've been hard to imagine it at the time.
Eric Topol (30:17):
Yeah. Well, when that gets simplified and doesn't require hospitalizations and bone marrow, and then you'll know you're off to the races. But look, what a great start. Phenomenal. So congratulations. I'm so thrilled to have the chance to have this conversation. And obviously we're all going to be following your work because what a beacon of science and progress and changing medicine. So thanks and give my best to my friend there at IGI, Fyodor, who's a character. He's a real character. I love the guy, and he's a good friend.
Jennifer Doudna (30:55):
I certainly will Eric, and thank you so much. It's been great talking with you.
*******************************************************
Thanks for listening and/or reading this edition of Ground Truths.
I hope you found it as stimulating as I did. Please share if you did!
A reminder that all Ground Truths posts (newsletter and podcast( are free without ads. Soon we’ll set it up so you can select what type of posts you want to be notified about.
If you wish to be a paid subscriber, know that all proceeds are donated to Scripps Research, and thanks for that—it greatly helped fund our summer internship program for 2023 and 2024.
Thanks to my producer Jessica Nguyen and to Sinjun Balabanoff for audio/video support.











Jennifer Doudna: The Exciting Future of Genome Editing