A turning point in cancer
A cluster of "un-heard of" results in multiple subtypes of advanced cancer
The narrative has been incubating for many years, but in recent days we are witnessing some extraordinary progress in treating and monitoring cancer. The convergence of genomics of the cancer—be it from the person’s DNA or tumor directly or the blood (known as liquid biopsy)—matched with the appropriate therapy is leading to outcomes that are being described as “unheard-of” by expert oncologists. This represents the essence of individualized medicine, whereby understanding the unique biologic basis of a person’s cancer can lead to highly accurate and effective treatment, and also avoid the toxicity of classical chemotherapeutic agents. Here I will review 4 recent studies, all published in the last week, and a new screening test that has become available.
Complete Remission in a Type of Advanced Colorectal Cancer
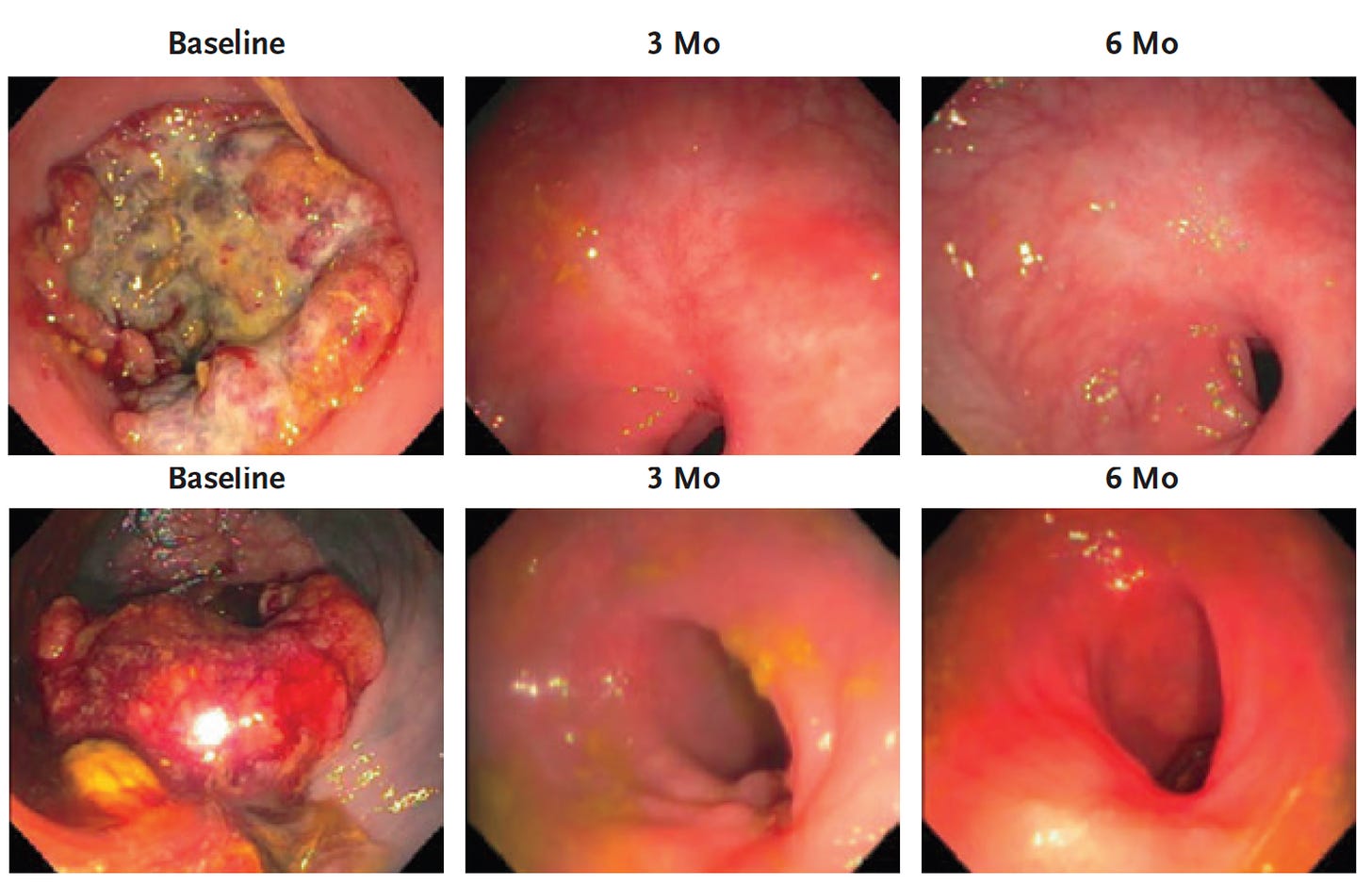
Mismatch-repair deficiency (MMR), a genomic diagnosis of mutations in genes that correct mistakes when DNA is copied in a cell, is present in up to 15% of people with colorectal cancer, but screening is woefully low. It is also found, at lesser frequencies, in many other cancers including breast, prostate, bladder, thyroid, uterus, and gastrointestinal. A new report of 18 consecutive patients with MMR, all treated with programmed-death (PD-1) blockade immunotherapy had complete remission of the tumor (12 in the paper and 6 additional in the media report), as represented below by serial, direct endoscopic visualization.
One of the authors, Dr. Diaz of Sloan Kettering, said ““I believe this is the first time this has happened in the history of cancer” while an outside expert at UCSF described it as “unheard-of.” Of course this is a finding that needs to be independently replicated with follow-up for potential recurrence. But it has to be seen as good news for individualized therapy of this subtype of cancer, in contrast to the current standard of care that involves chemotherapy, radiation and surgery that only achieves complete remission in 1/4th of patients. Furthermore, there are an extensive list of serious complications of this usual approach. The alternative here, immune checkpoint blockade, carries far less potential of toxicity. Indeed, in the current study only minor side effects such as a rash or nausea were reported.
Improved Survival in a Type of Metastatic Breast Cancer
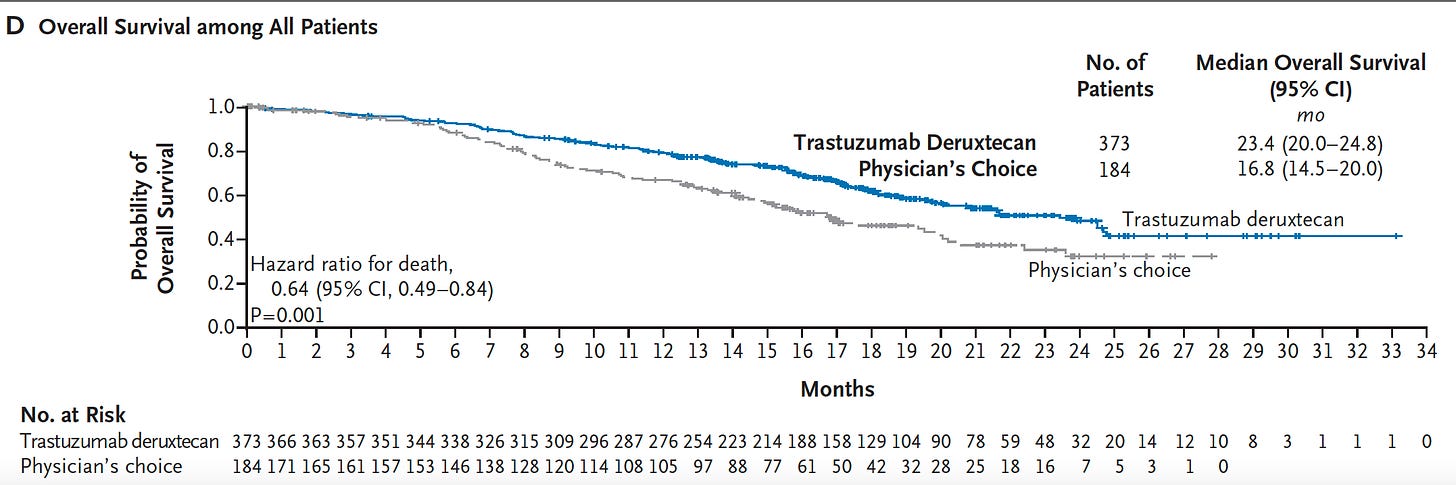
A randomized trial in women with low human epidermal growth factor expression (“HER2-low”) metastatic breast cancer was published last week comparing physicians choice with the drug trastuzumab deruxtecan, a monoclonal antibody that targets tumor cells with low levels of HER2 expression. This is one of the first clinical trials dedicated to HER2 low. The results were striking for this clinical condition as illustrated by the impact on overall survival shown here:
A breast cancer specialist, not involved in the trial, declared “This is a new standard of care. It affects a huge number of patients.” A co-author said “It is unheard-of for chemotherapy trials in metastatic breast cancer to improve survival in patients by six months.” Here is the link to the coverage in the New York Times along with the text of the article shown below. Unlike the immune checkpoint blockade in the prior report, withs its relatively benign side-effect profile, this is a chemotherapy which has significant side effects, such as the potential of inducing interstitial lung disease.
Engineering the T cells for a Patient with Metastatic Pancreatic Cancer
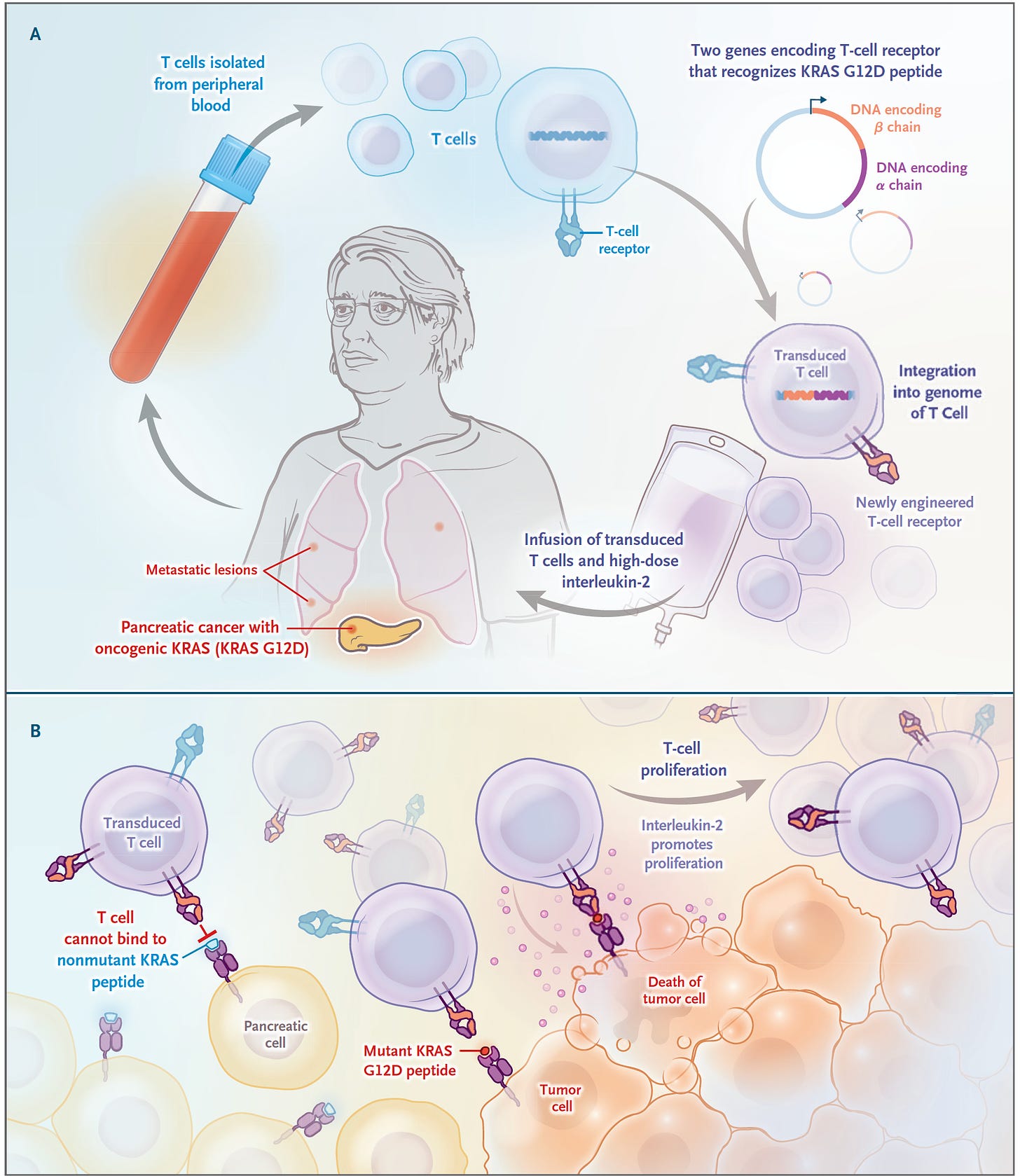
It’s just a case report but certainly a groundbreaking one for the deadliest form of all common cancers—pancreatic ductal adenocarcinoma. 90% of these cancers have a mutation the KRAS gene, and G12D is the most common one, in about one-third of such cancers. In June 2021, a 71-year-old woman with recurrence of pancreatic cancer that had been extensively treated (including a Whipple surgical procedure, radiation and multiple agent chemotherapy) presented with new lung metastases, received a single infusion of her blood with engineered T cells with 2 genes encoding T cell receptors, that were directed to both the G12D mutation and an HLA allele (HLA-C*08:02) of the immune system, as shown below
The results of marked regression of her tumor were extraordinary and persistent, although not shared by another patient similarly treated who succumbed from this cancer. While engineering a person’s T cells (chimeric antigen receptor, CAR-T) has been effective for blood cancers, this was a notable report in a refractory patient with metastatic pancreatic cancer. And KRAS mutations in various cancers have had 2 decades of immunologic treatments without prior success.
Liquid Biopsy Monitoring of Colorectal Cancer to Reduce/Avoid Chemotherapy
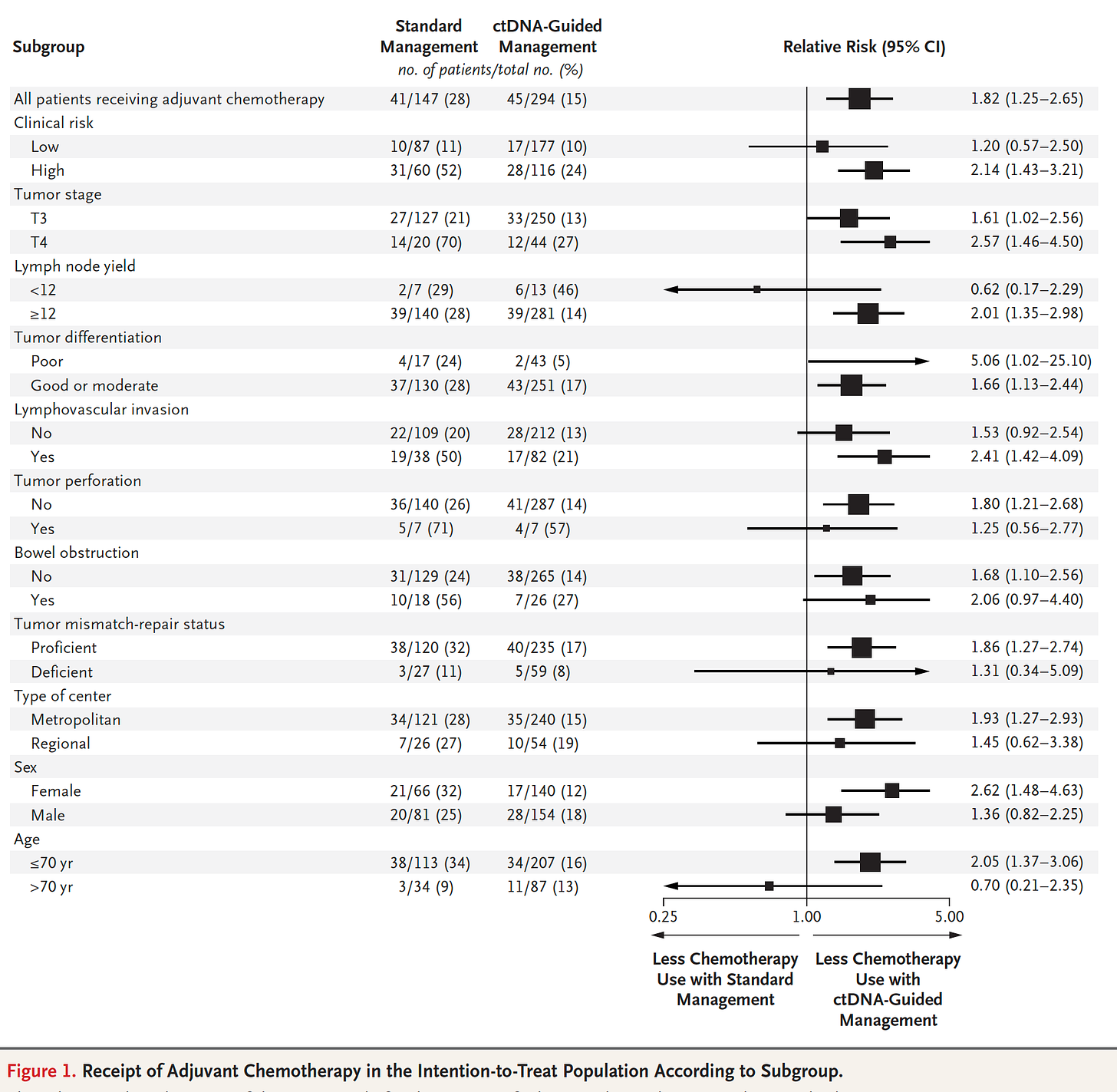
In a trial of liquid DNA biopsy in Stage II colon cancer, 455 patients were randomly assigned to cell-free tumor DNA (ct-DNA, liquid biopsy) management and 153 patients to standard care with over 3 year median follow-up. The ct-DNA group had substantially less (halving) need for chemotherapy without any reduction of cancer-free survival, which was a consistent benefit across nearly all subgroups as shown here
The accompanying editorial framed this well: “the results provide a solid foundation for a new way to personalize adjuvant chemotherapy for colon cancer—one in which biology outperforms anatomy to improve therapy.” That is, the ct-DNA guided management of patients away from unnecessary chemotherapy with all of its toxicity, and even potential of untoward, mutagenic (inducing mutations) effects. There are many other ongoing trials like this one that will likely confirm the ability to use a liquid biopsy to guide individualized chemotherapy for not just colon cancer, but a variety of other solid tumors. In a related report, using a liquid biopsy using a biomarker for PD-L1 (from extracellular vesicles) was superior to tumor biopsy of the lung for guiding immune checkpoint inhibitor therapy This was the first report for a blood test found to be better than an invasive tumor biopsy to guide immunotherapy.
The First Liquid Biopsy Cancer Screening Test
The company GRAIL launched a liquid biopsy test called Galleri, which the company says “is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older. It is intended to be used in addition to and not replace other cancer screening tests your healthcare provider recommends.” It costs $949. Unlike many tests that focus on the DNA sequence changes, this test assesses changes of methylation at key sites across the genome and uses a machine learning algorithm of the methylation pattern to predict the origin of the cancer. In 2 research reports, more than 50 types of cancer have been detected with a 0.5% false-positive rate and the primary origin of the cancer was identified 89% of the time. The overall sensitivity for stages I-III of cancer was low at 41%. In discussing this test with the GRAIL chief medical officer this week, over 30,000 have now been done in clinical practice. For the first 10,000 people, 1.1% were positive for cancer and of the 114 people where a cancer signal was detected, 71% had the origin accurately localized.
There is an ongoing randomized trial in the UK of 150,000 people age 50+ with risk factors for cancer which is assessing whether this test changes outcomes.
I do not recommend this test but write about it here because it is starting to attract considerable attention as a new way to screen for cancer. However, that is not yet clinically validated (or approved by FDA) and it may be that a person has a cancer signal, but on repeat testing a month or two later, the signal is gone—not a “false positive” but rather the impact of a person’s healthy immune system responding to a threat of cancer at the microscopic level. The worry about false positives extends to localizing a cancer when there is a positive , which could necessitate total body scanning with either PET or other imaging modalities, no less the profound anxiety (and cost) as the work-up proceeds. These uncertainties, and the false negative issues from low sensitivity, also should be placed in context of using only 1 layer of data—the targeted methylation pattern. The DNA sequence with its mutations, copy number, structural variants—are all ignored, along with extracellular vesicles and other informative biomarkers. Another company called Delfi uses artificial intelligence of the ct-DNA fragments to detect and localize cancer. Other companies such as Exact Science and Freenome are getting ready to launch screening tests for ct-DNA, with or without some specific protein assays. None of these companies have coupled their tests with polygenic risk scores (which have been previously validated for colon, breast, prostate, and other cancers) or genome sequencing for cancer predisposition genes, both of which could help identify high risk individuals and enhance the predictive value of the liquid biopsy results.
But you can see where this is ultimately headed, which is potentially quite exciting. From a tube of blood (or 2 in the case of the Galleri test), all these layers of data could be ascertained, processed with advanced analytics, with the result of signal detection and localization that would be highly accurate in the years ahead.
We dread the diagnosis of cancer, not only because of its threat to life, but also the conventional chemotherapies that are given, with considerable toxicity. But the theme of the clusters of individualized medicine I’ve reviewed here offers a way forward that links biology and therapy. That reduces the need for chemotherapy. Moreover, as plasma cell-free tumor DNA tests get more informative, the dream of the earliest possible diagnosis of cancer may ultimately be fulfilled, and coupled with an individualized, biologic-based treatment when necessary. So at the very least, I hope you’ve now heard of some “unheard-of” important, new results that foster considerable hope for better cancer outcomes in the future.
Those of you who have been following my substack posts over the past 6 months will realize this is the first one that is not Covid-centric. When I started Ground Truths my intent was to get back to my primary area of interest—individualized medicine, using genomics and digital tools with AI analytics. That is certainly my plan going forward, although I will intermittently be returning to Covid matters as issues crop up.
Thanks for reading. Subscriptions are free and I encourage you to join the list to get notifications of a new post, and share the post if you found it helpful.