Just think of the implications:
“A new type of vaccine…has shown in the lab setting that it can completely reverse autoimmune diseases like multiple sclerosis and type 1 diabetes—all without shutting down the rest of the immune system.”
We’ll get to “Leveraging the Liver,” as depicted below, after some background.
Tolerogenic vaccines are the opposite of conventional vaccines that rev up our immune system against a pathogen. Instead, they suppress or block our immune response. Inducing tolerance—just what we need for people at risk for or suffering from autoimmune conditions. The tolerogenic concept isn’t new and has been intensively pursued for at least 15 years.
Current treatments for the more than 80 autoimmune diseases (multiple sclerosis, rheumatoid arthritis, celiac disease, lupus, psoriatic arthritis, to name a few) affecting more than 5% of the population involve blocking the immune system with drugs, which lack specificity and leave people vulnerable for opportunistic infections, malignancies, and side effects. Furthermore, their efficacy is often limited as seen with refractory cases and they are by no means preventative or curative. Moreover, the duration of therapy is typically lifelong.
The complexity of developing better approaches is formidable with the interplay of genetics, epigenetics, environmental triggers, and different, multiple (self-directed) autoantigens in any given individual as underpinnings for their condition. Nonetheless, there have been many candidate vaccines in experimental models that have been published.
Prior Work with Tolerogenic Vaccines
For example, in 2021, the BioNTech group published in Science a mRNA vaccine in the mouse model of multiple sclerosis known as autoimmune encephalomyelitis. In this model, a specific antigen drives the disease and induces progressive damage to neural tissue. As schematically shown below, using that antigen, myelin oligodendrocyte glycoprotein peptide (MOG), and an adjuvant, the mRNA-based vaccine lipid nanoparticle complex (RNA-LPX) with 1-methylpseduouridine (m1Ψ), compared with suitable controls, markedly dampened the immune response with stimulation of suppressive Treg (reg=regulatory) cells and less inflammatory cytokine mediators. The vaccine prevented disease in mice when given 7-10 days in advance, and there was tissue evidence of reduced demyelination in the spinal cord and brain.
But that approach has many limitations. Multiple sclerosis is driven by many different autoantigens and a complex polyclonal T cell repertoire, varying from one person to the next. The immune suppression of m1Ψ mRNA vaccination is generalized. Whereas the model is a single hit of autoantigens, the disease process in people is thought to be due to continuous exposure of autoantigens.
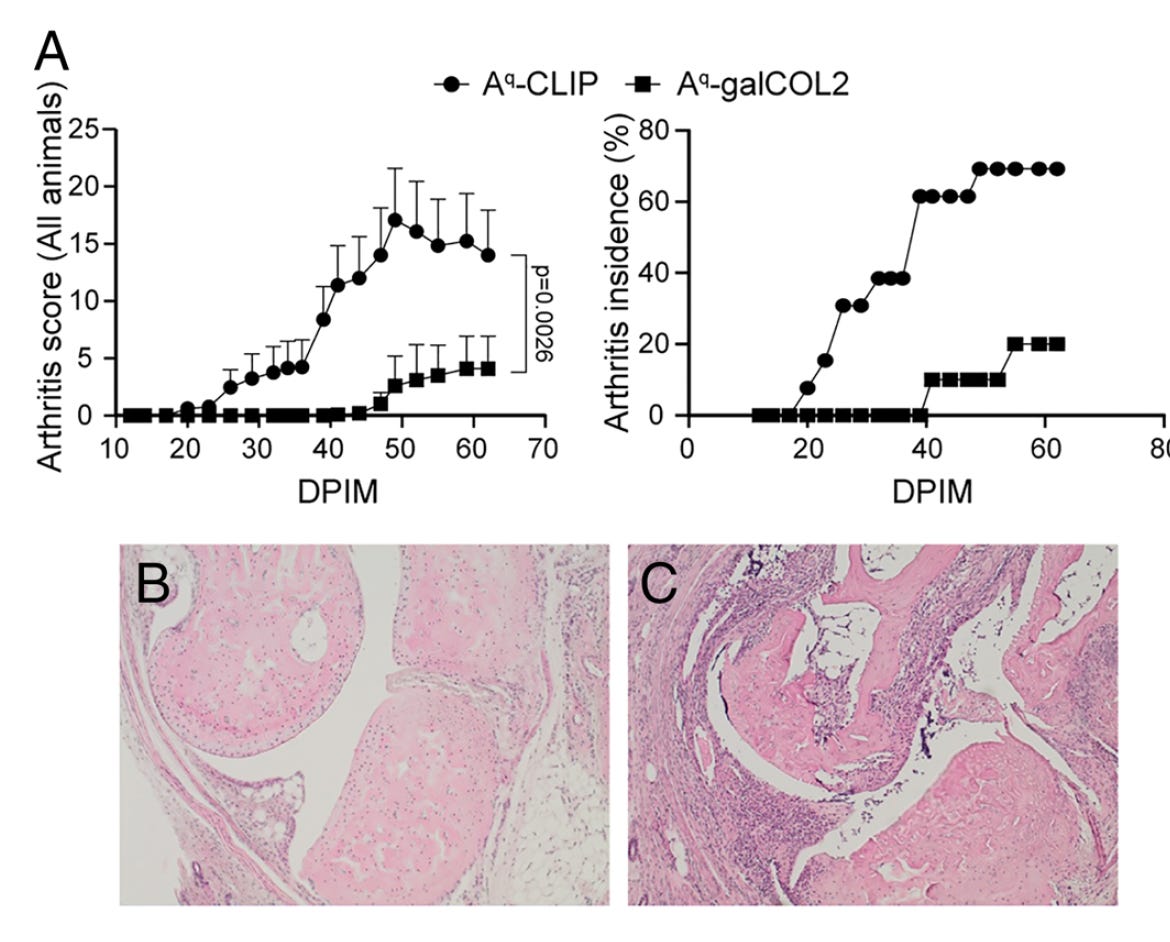
In other prior vaccine work, in a mouse model of rheumatoid arthritis administration of an autoantigen bound to a glycosylated peptide (galCOL2), had a potent effect of stimulating Treg cells and protecting against arthritis.
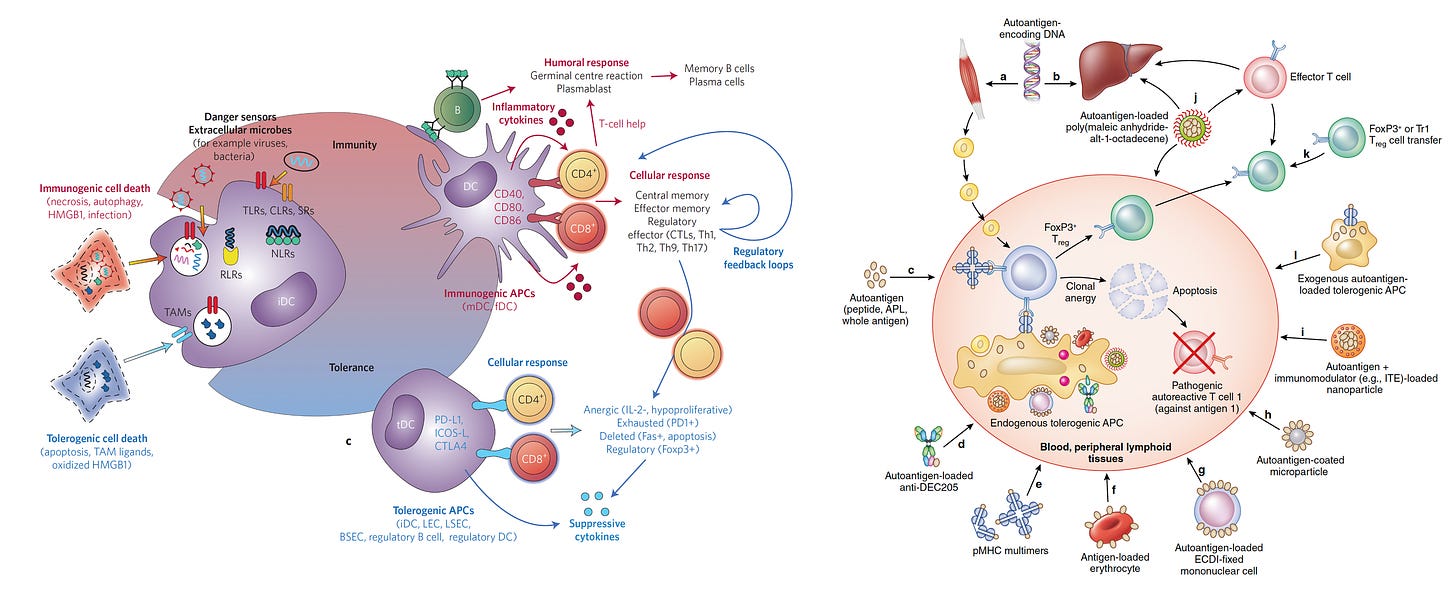
The search for optimal ways to achieve immune tolerance (lower half of left panel) and blocking autoantigens (right panel) has been long and hard, and I won’t delve into the complexity here but refer you to the links for these 2 very good review papers. Full disclosure: I should note that Klaus Ley and I are co-founders of a very early start-up company called Atherovax, which someday we hope to develop a tolerogenic vaccine to block atherosclerosis in people at high risk.
Leveraging the Liver
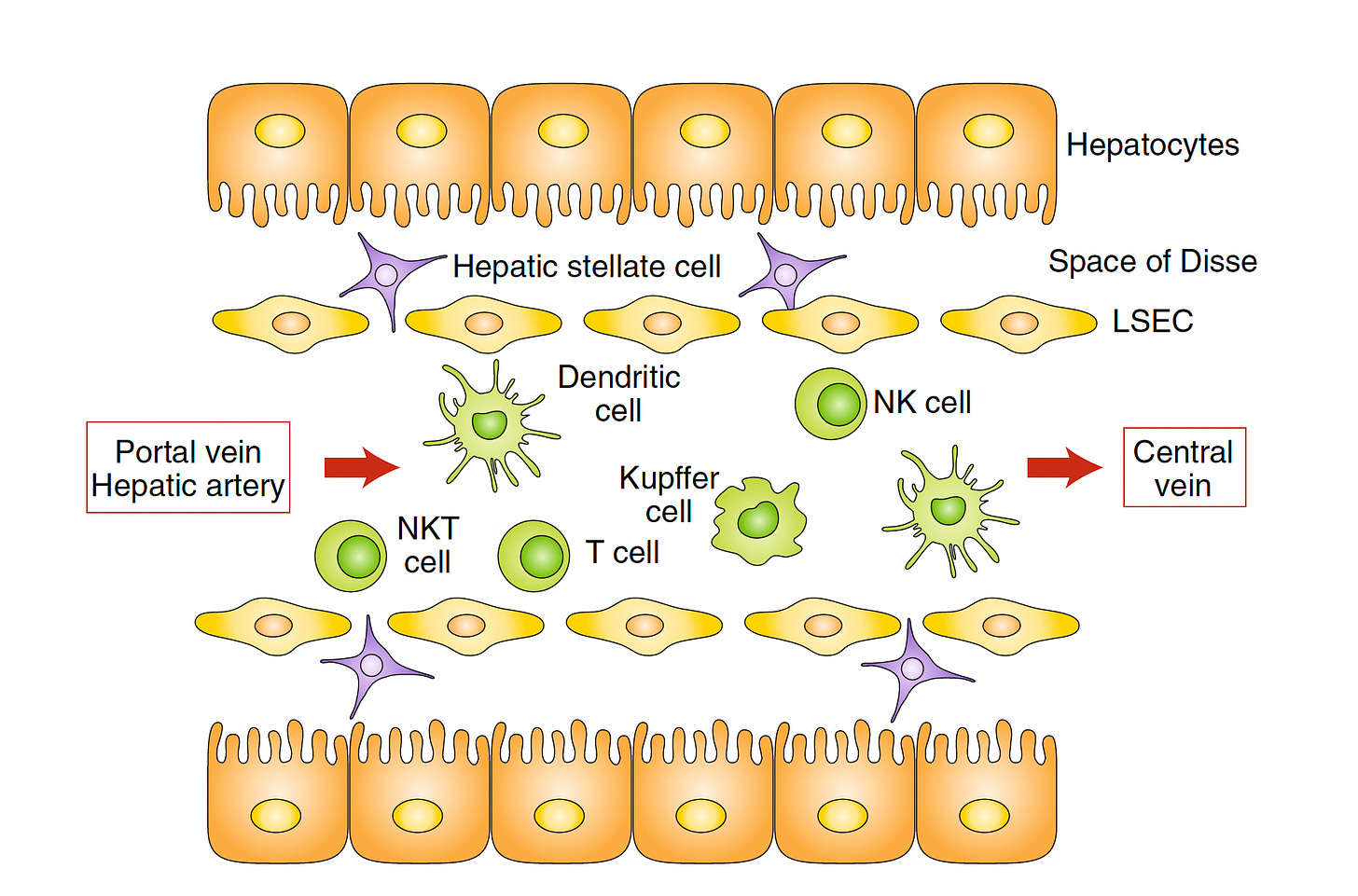
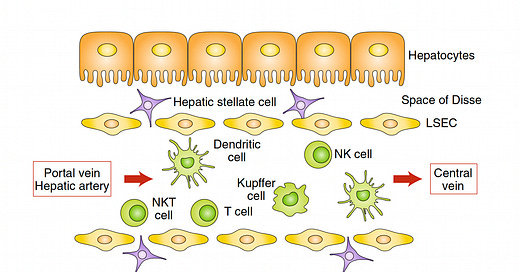
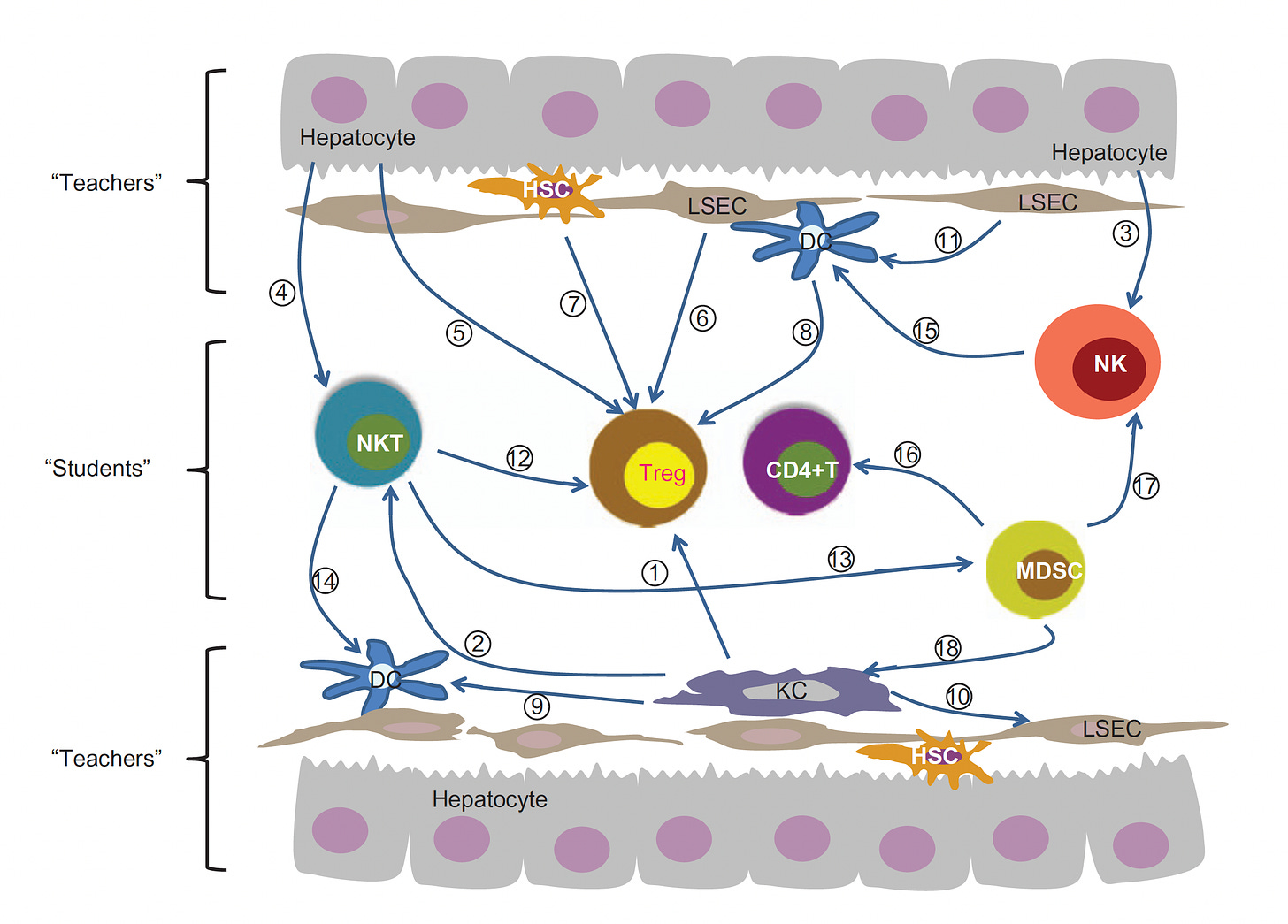
A decade ago a paper entitled “The Liver Works as a School to Educate Regulatory Immune Cells”, with the graphic below, foreshadowed the approach that Jeffrey Hubbell and colleagues at University of Chicago would take. The idea is that the liver is a unique tolerogenic environment and can be the school for circuiting immune cells (students) and its resident antigen-presenting cells (HAPCs) serving as teachers.
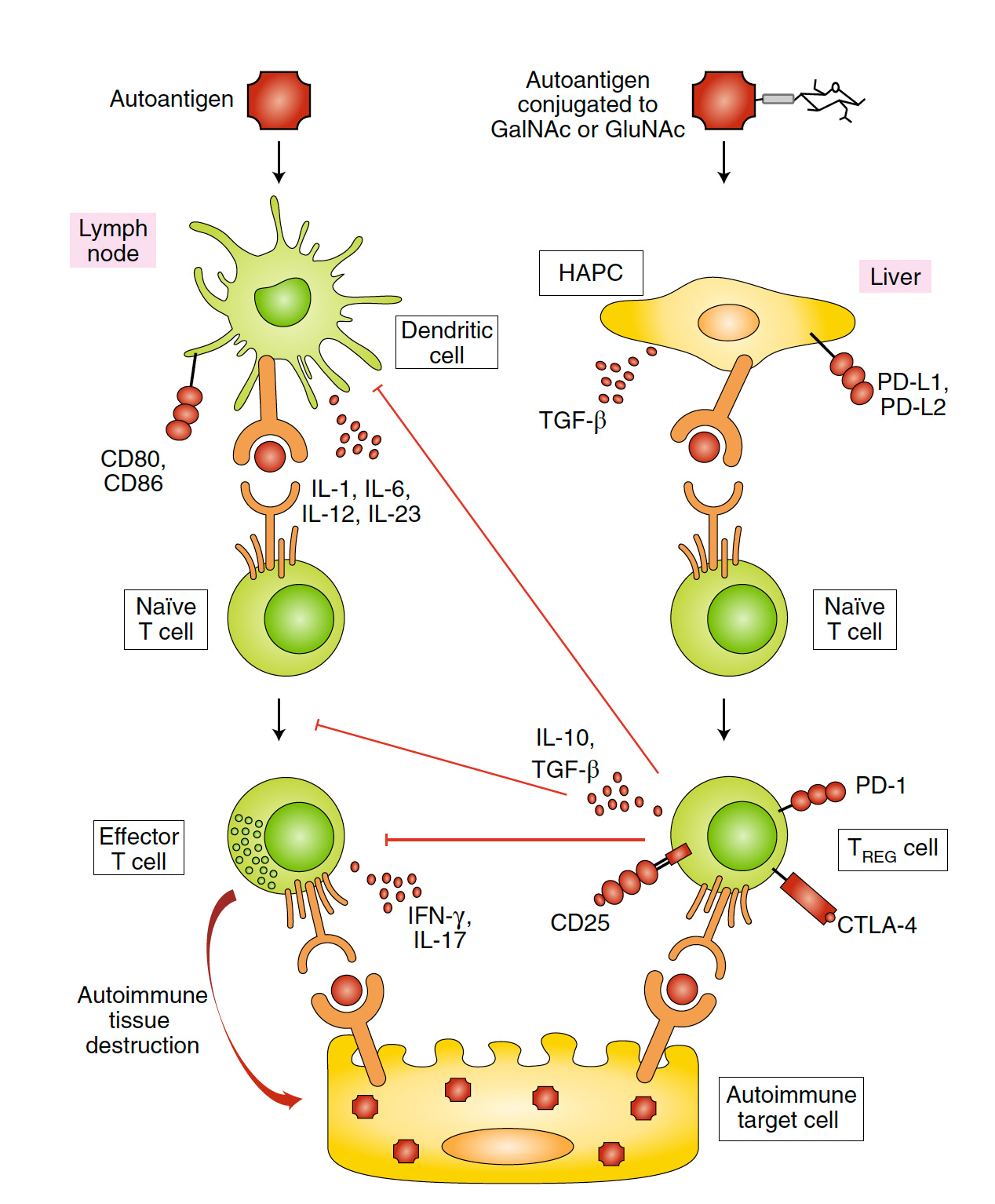
In 2019, Hubbell and colleagues published a paper on synthetically glycosylated antigens (added sugar molecules) to prevent type 1 (autoimmune) diabetes in the experimental mouse model. Taking advantage of the “liver’s tolerance-inducing machinery” autoantigens were targeted to HAPCs to induce antigen-specific tolerance and Treg memory. By glycosylation (with either GalNAc or GluNAc), the autoantigens can be modified with the HAPCs and the prevention of T-cell mediated diabetes, as shown below.
However, like the mRNA vaccine approach reviewed above, this was to prevent the autoimmune condition, not to treat or cure it.
But now, in a new paper by Hubbell’s group, 2 different models were assessed with this glycosylation approach, including the multiple sclerosis (autoimmune encephalomyelitis) and antigen-specific suppression of simian immunodeficiency virus vaccine in non-human primates. They dubbed this as “inverse vaccines” because of their objective being the opposite of conventional vaccines that stimulate the immune response, although that term has been used previously by others. They offered a simple explanation for how this works: ‘The liver naturally marks molecules from broken-down cells with “do not attack” flags to prevent autoimmune reaction to cells that die by natural processes. [The glycosylation] coupled an antigen with a molecule resembling a fragment of an aged cell that the liver would recognize as friend, rather than foe.’
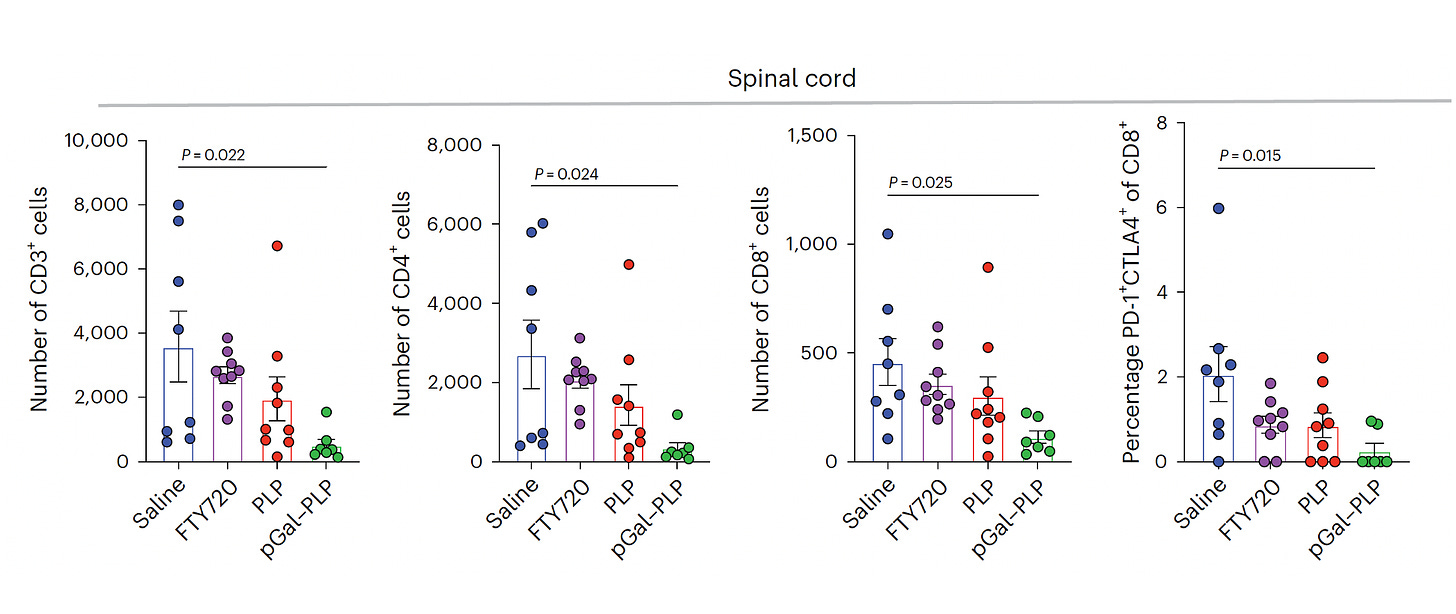
The advance here was the demonstration as an effective treatment, not just a prevention. The team showed in the multiple sclerosis model that glycosylated myelin proteins stopped neural damage and reversed symptoms (Figure below for spinal cord; PLP is the myelin antigen, and pGal-PLP is the glycosylated antigen). Furthermore, there were indicators of an otherwise intact cellular immune response, rather than global suppression that is induced by drug therapies.
This work has now led to a Phase 1 trial report in celiac disease in 41 patients with an acceptable safety profile and on ongoing trial in multiple sclerosis. It is important to point out that Hubbell and some of the co-authors’ colleagues hold patents on this approach and hold positions in the company Anokion SA with equity interests, and that Anokion provided some funding for this work.
Summing Up
The approach of leveraging the liver to induce immune tolerance is intriguing and the new published work takes this a step further as a potential treatment in the future. There are still plenty of uncertainties, such as knowing the autoantigens in a particular individual or fully understanding the mechanism of tolerance induction. A tolerogenic vaccine is a huge challenge and orders of magnitude more complex than stimulating the immune system against a particular pathogen. There’s already a graveyard full of failed tolerogenic approaches and it’s certainly possible that glycosylation and the liver pathway could ultimately fail too. Nevertheless, I would deem it as an exciting approach—if it works, it will have a transformative impact on a wide array of self-destructive medical conditions for which we don’t yet have highly effective and safe means for preventing or treating.
Thanks for reading and subscribing to Ground Truths!
Please share this post if you found it informative or helpful.



Is it possible that this new type of vaccine might be of use in reversing the almost stochastic symptoms of autoimmune responses associated with long-Covid?
Is there any hope that writing about and researching Lyme Disease will grab an interest in the medical world?
One of the very prevalent issues today is “WHY” is there such a hush hush about admitting that Chronic Lyme disease exists?
Believe me, having been living with it since 1985 I know more about it and what it can do to ruin one’s’ immune system than most medical professionals. Why is that?
Is it in fact because our very own government used ticks to work as bio-terrorism.?