Cancer, the Master of Hijacking
Discoveries of the ways cancer commandeers our cells and tissue keep expanding, as do potential treatments to counter
“Cancer doesn’t really invent anything new— it just hijacks processes that already exist.”—Michelle Monje
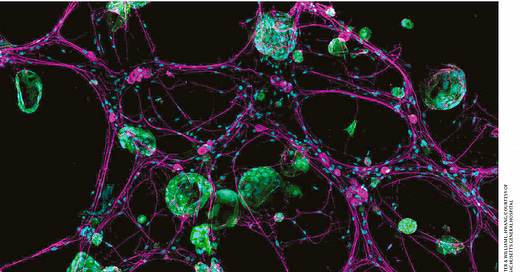
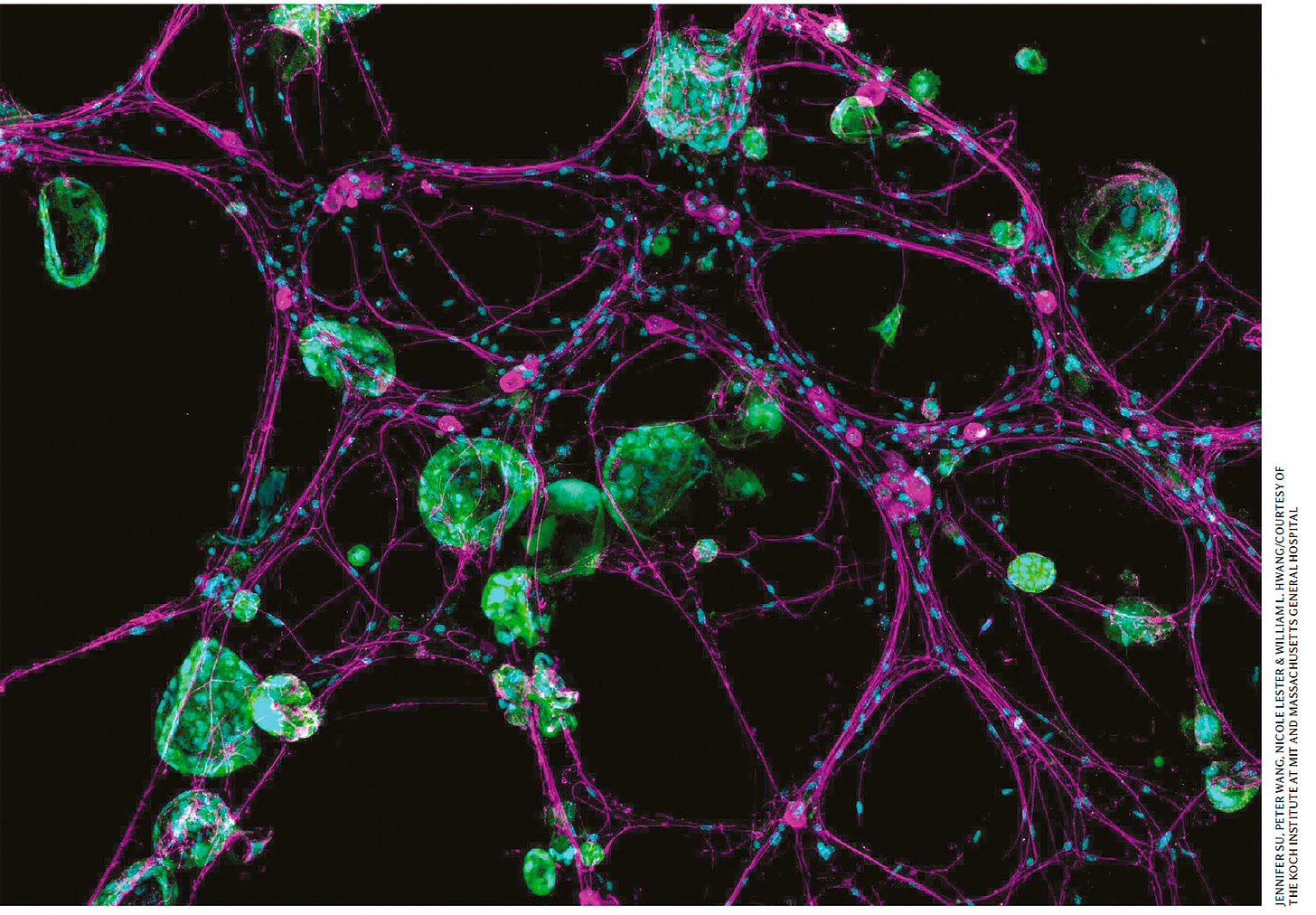
Cancer cells in green, hijacking the neurons in purple, from Koch Institute, at Nature
Hijacking Lipids
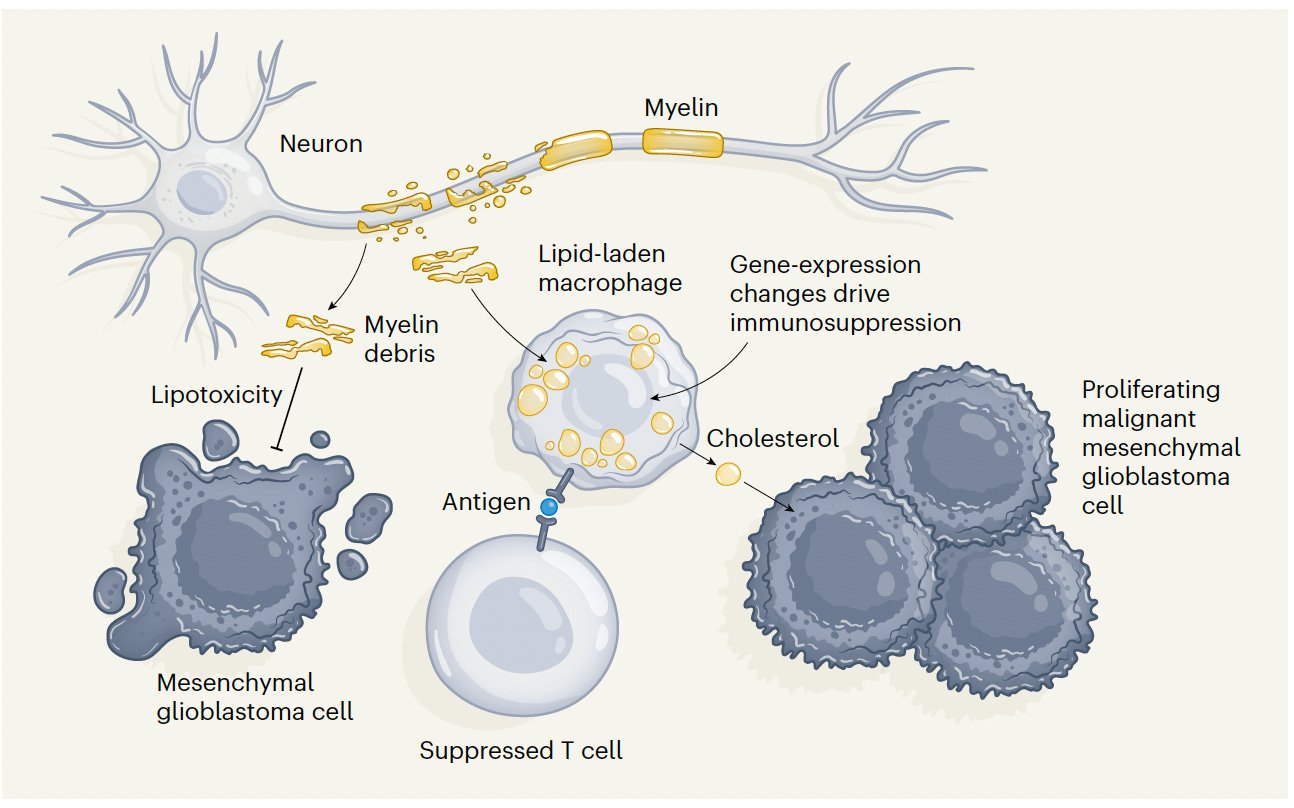
Recently, we learned about another type of LLM—lipid-laden macrophages—and their role in brain cancer (not large language models!) The resident macrophages of the brain, known as microglia, and monocyte-derived macrophages, form LLMs by scavenging cholesterol lipid from the myelin sheath of neurons, as schematically shown below.
As a result, there’s a triple whammy. Not only do the LLMs suppress our immune response to the cancer, such as by changes in gene expression and blunted T cell capacity, but they transfer cholesterol to the mesenchymal glioblastoma cells which enhances their proliferation. Beyond that, if the myelin debris was not picked up by LLMs, it would otherwise have toxic effect to limit tumor progression. Unraveling this mechanism raises the potential of many therapeutic approaches to target and manipulate LLMs (such a blocking lipid-exporter proteins), which led to survival benefit in a mouse model but has yet to be tested clinically.
But this lipid mechanism is just the latest of many ways by which cancer cells show their opportunistic capabilities. Let’s review the others.
Hijacking Neurons and Neural Circuits
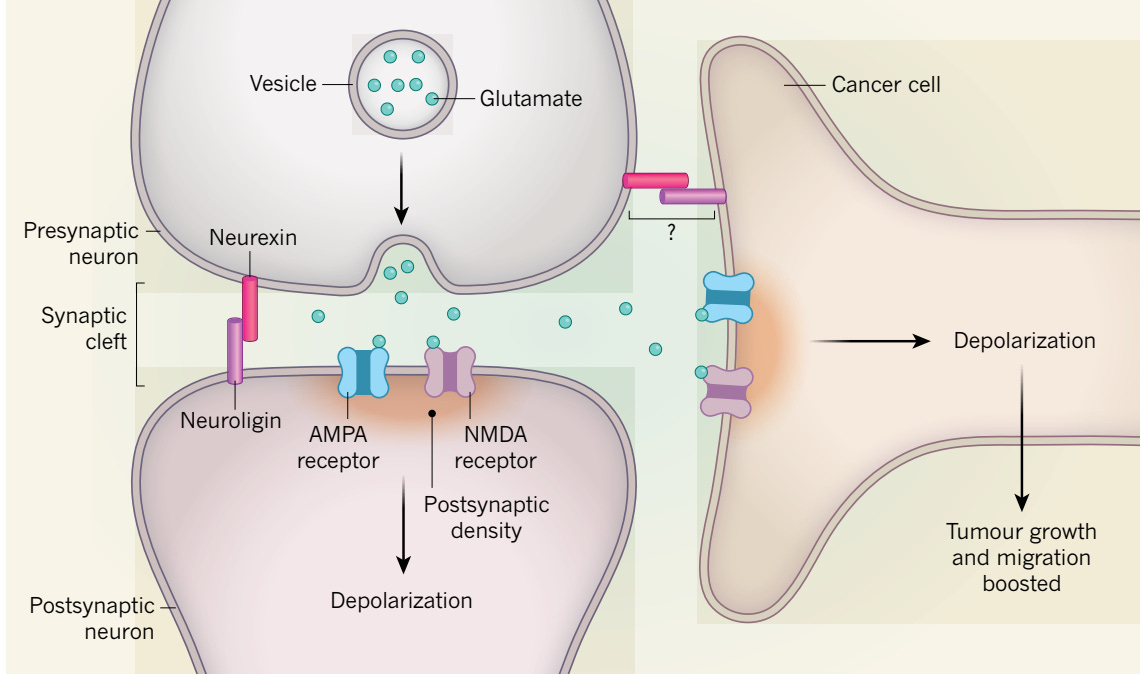
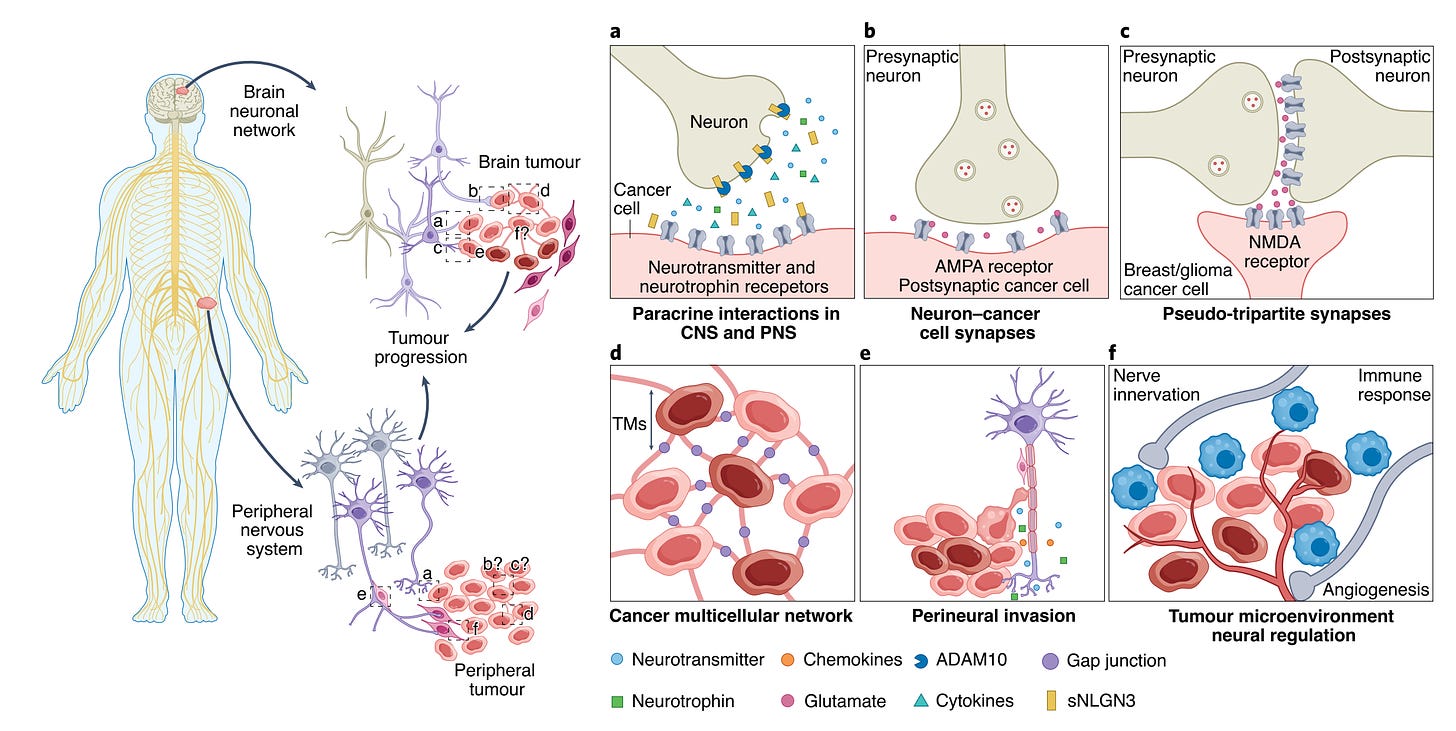
Back in 2019, also in brain cancer, Venkataramani and colleagues, Venkatesh et al, and Zeng et al, simultaneously reported that direct synapses were formed between neurons and cancer cells. The functional significance of these synapses, as shown below, is to promote tumor growth and migration. Although some connections between cancer and neurons had between reported many years previously, as reviewed here, the 3 papers laid the foundation for the field of cancer neuroscience. In my Ground Truths podcast with Michelle Monje, she reviewed how this field has blossomed.
It turned out that was only the first chapter to the story. The hijacking of the nervous system goes beyond direct synapses, and can be invoked by neurotransmitters and nerve growth factors forming membrane tube connections in the brain, spinal cord, and peripheral neural tissue (graphic summary below).
Gene mutations in other cancers, such as P53-driven head and neck tumors, have been shown to reprogram nerve cells, enabling their secretion of epinephrine and norepinephrine, that stimulates tumor growth. And the cancer-induced reprogramming of neural cell beta-adrenergic receptors promotes T-cell exhaustion, again compromising our immune response or immunotherapy to fight cancer. These insights have led to many cancer treatment trials targeting neural pathways, such as with beta blockers, clonidine, anti-seizure meds, or botulinum toxin.
While the initial discovery of neuron-cell connects was in brain cancer, tumor innervation has now been shown to worsen patient outcome across multiple types of cancer. Recently, it was shown that breast cancer cells can induce release of neuropeptide substance P from sensory neurons, a molecule typically associated with pain and inflammation, and that an approved anti-nausea drug (aprepitant, Emend) suppressed breast cancer growth and spread in multiple experimental models.
Hijacking Mitochondria
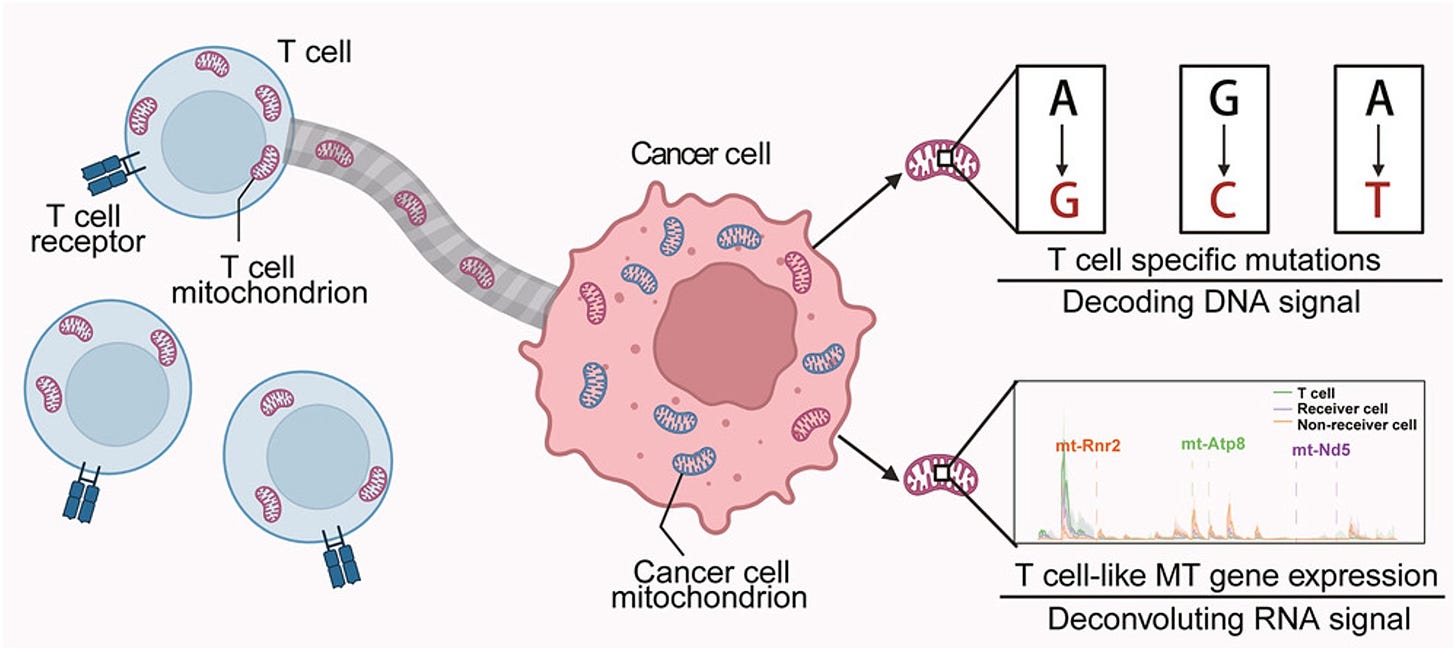
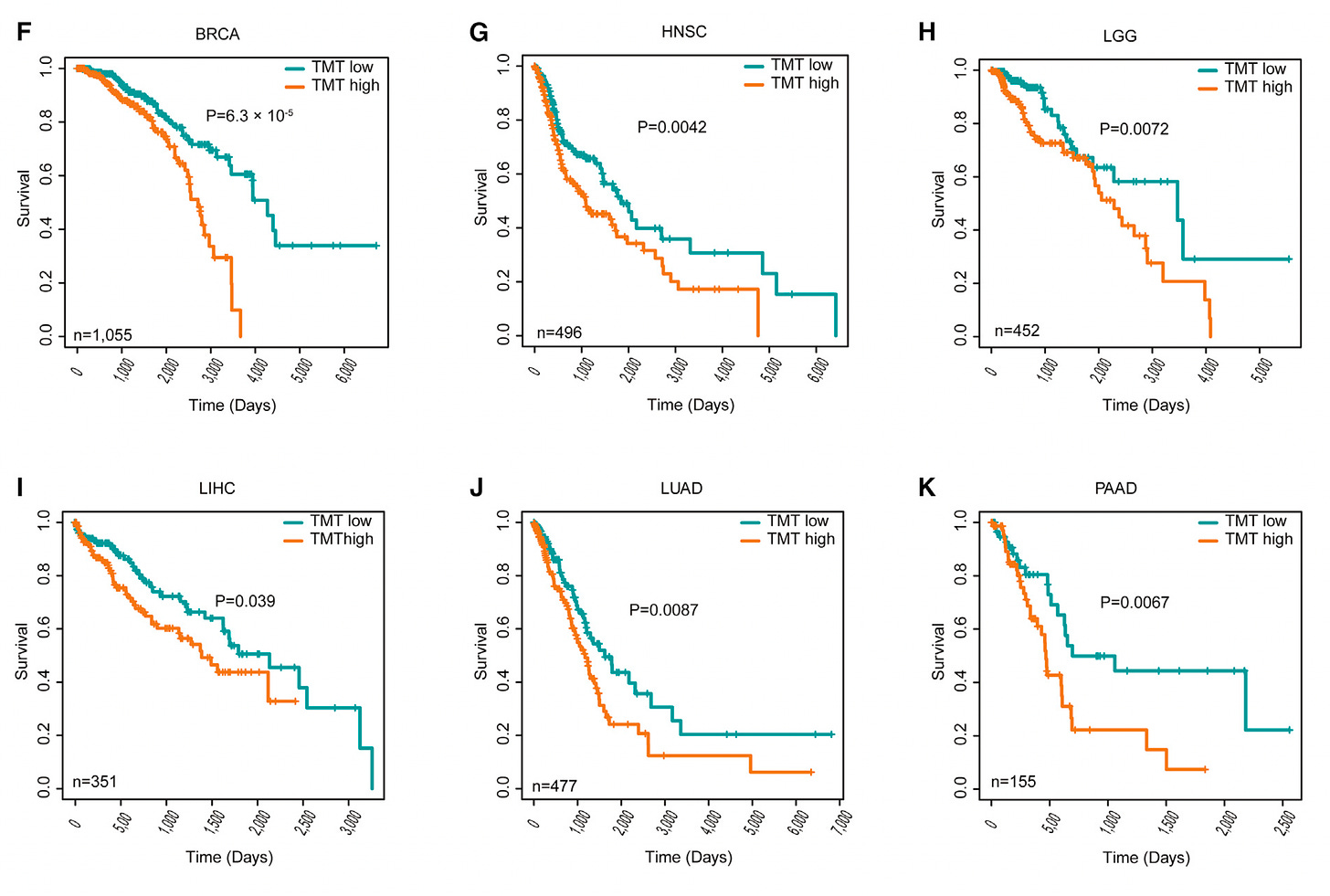
As demonstrated by elegant single-cell resolution, cancer cells hijack the mitochondria from T cells! This is remarkable because it simultaneously takes down the immune response to the tumor and empowers cancer cells with their stolen powerhouse fuel supply. Indeed, when mitochondrial transfer has been documented in patients; it is associated with worse clinical outcomes across multiple cancer types.
The clinical impact across 6 different types of cancer, TMT-tumor to mitochondrial transfer. This discovery again has led to potential new therapeutics, since this transfer occurs through tunneling nanotubes; their formation may be blocked via drugs such as farnesyltransferase inhibitors and cytochalasin (an idea not yet tested in experimental models).
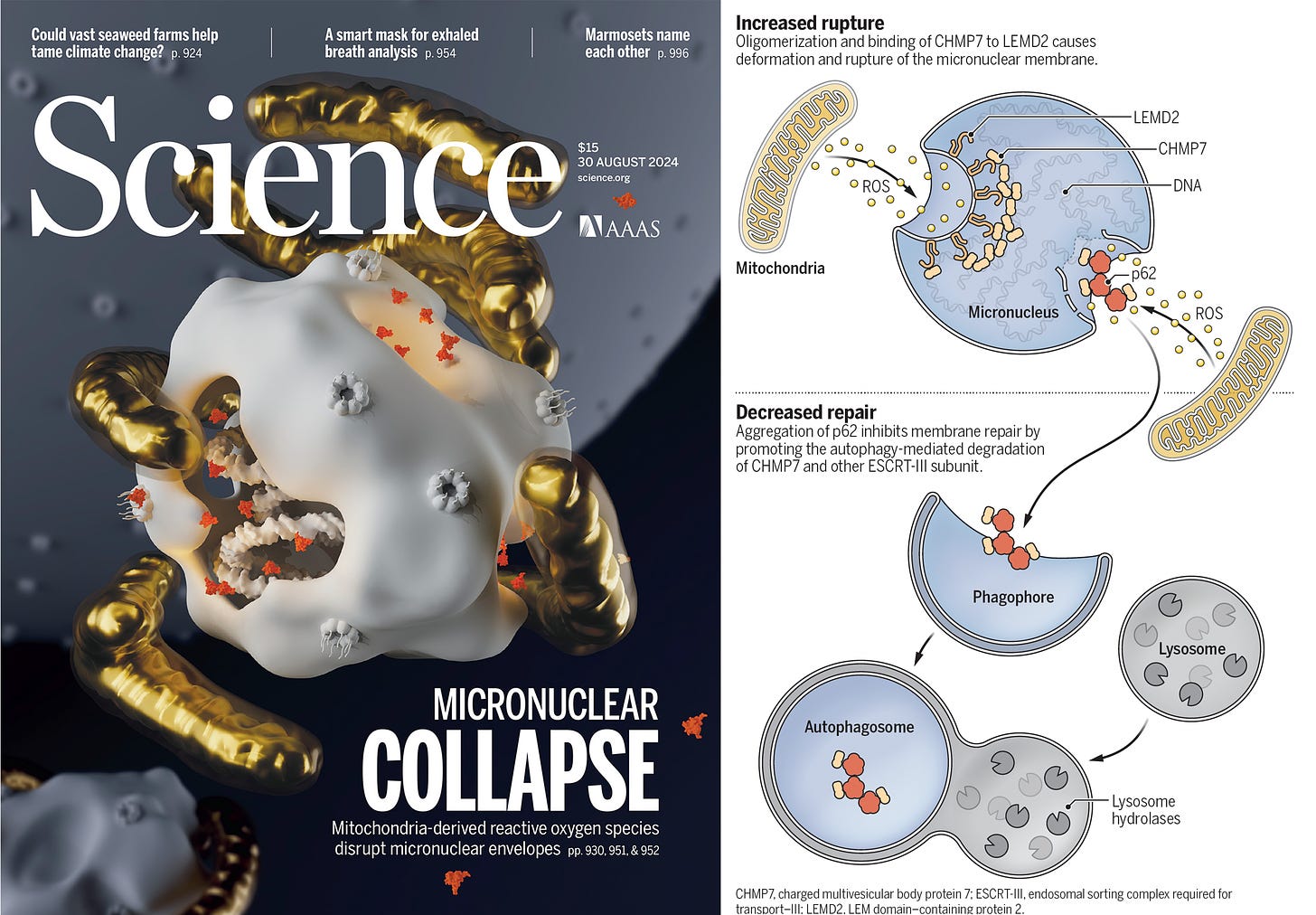
And while we’re on the mitochondria, it’s hard to resists the multiple reports (here, here and perspective here) in a very recent issue of Science, showing the link between the mitochondria and micronuclei collapse in cancer. These are small nuclei in the cytoplasm of the cell which can breakdown as a result of mitochondria oxidative damage, release their DNA, induce genomic instability, and lead to cancer progression.
Hijacking Macrophages and Other Immune Responses
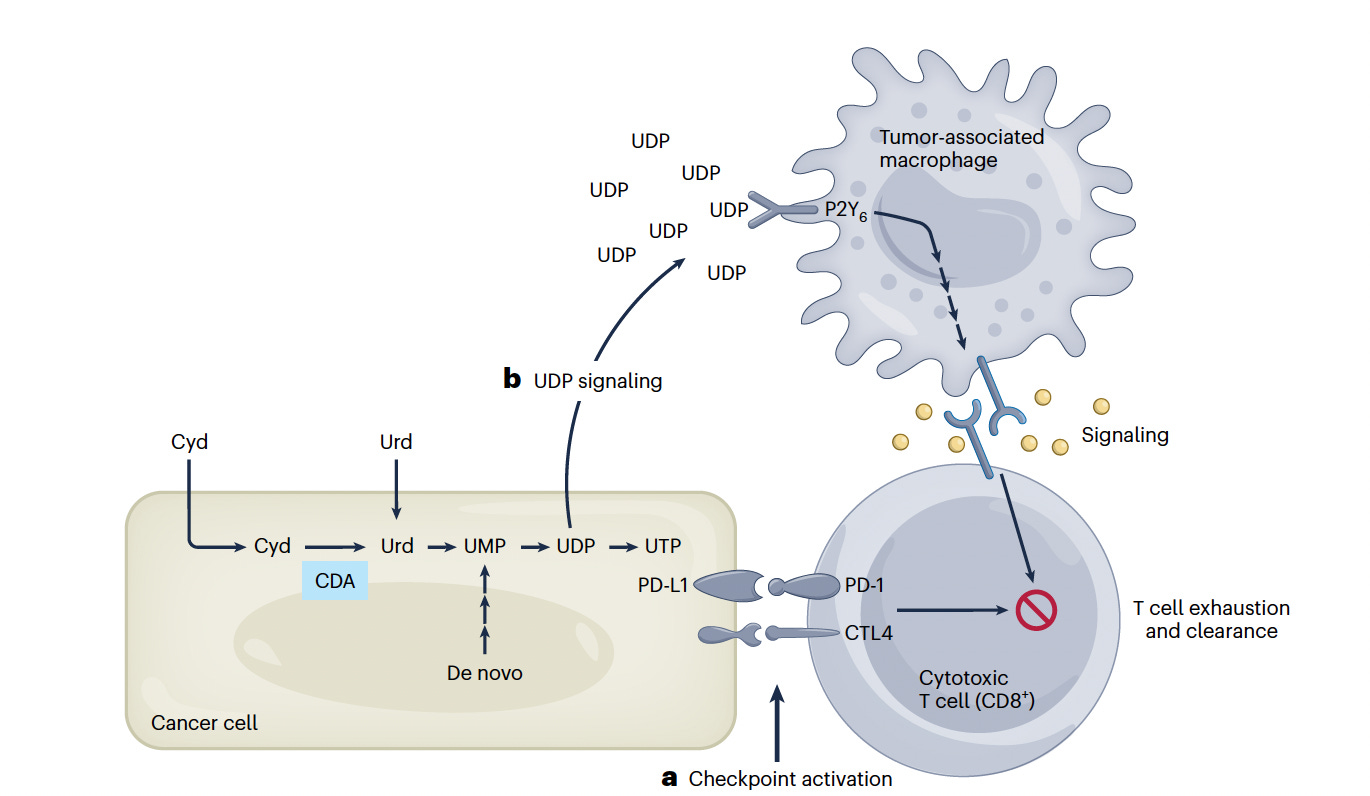
Many cancers are resistant to immune checkpoint inhibitors. Cancer cells express checkpoint inhibitor ligands, which can account for resistance to cytotoxic (CD8+) T cells. Beyond this, as demonstrated in pancreatic cancer, over-expression of cytidine deaminase (Cyd) leads to high uridine diphosphate levels (UDP),attracting immunosuppressive macrophages and creating an immunoprotected sanctuary. The finding has raised the potential for treating with an inhibitor of Cyd or to target the macrophage receptor (P2Y6), to block the immune evasion.
There are multiple other ways that cancer has been shown to hijack our immune response, such as the cells producing lactate that activates a protein called SREBP2 within dendritic cells (DC) which reprograms these key cells (into“ mregDCs”) which act as traitors, suppressing the body’s immune response to cancer. There’s also cancer cell rewiring of the STING pathway, a key immune system response.
Hijacking Blood Vessels
The cancer takeover of blood supply or its modulation has long been established, via release of angiogenic (blood vessel creating) growth factors (such as vascular endothelial growth factor, VEGF), growing into the existing blood cells, constricting neighboring blood vessels and inducing leaks that promote cancer spread, or via programmed cell death of endothelial cells lining blood vessels. There are several anti-angiogenesis drugs that have been FDA approved (such as bevacizumab, Avastin, sunitibib, Sutent, and soafenib, Nexavar) for various cancers, typically used in combination with other chemotherapy, but thus far have achieved fairly limited and variable success.
Catch Me If You Can
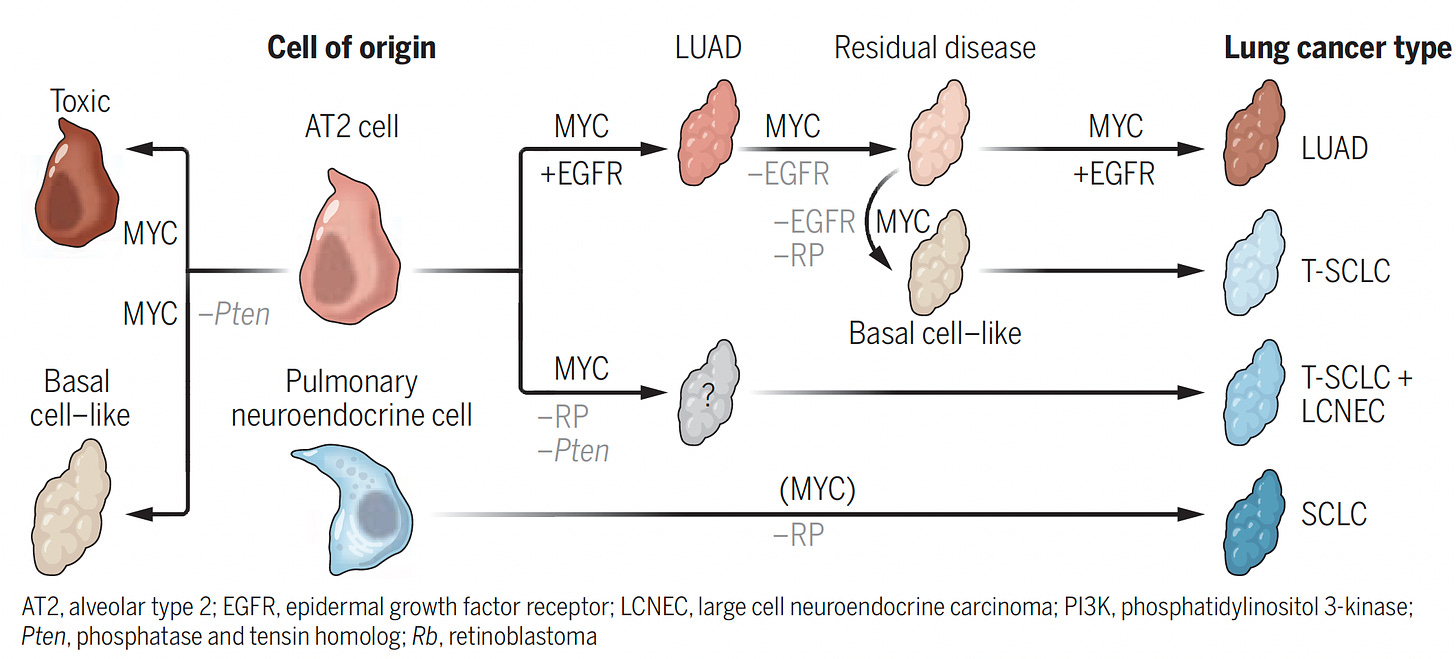
While not exactly considered hijacking, the recent finding that cancer cells can actually change their driver mutations and cancer type, such as from lung adenocarcinoma to small cell lung cancer, evading targeted therapy, fits into the overall pattern of deception and evasiveness, and schematically shown below.
Summing Up
I’ve tried here to take you through some of the numerous (and fascinating) ways that cancer can take over various types of cells, sub-cellular components (mitochondria), and tissue (neural, blood vessel) in our body, evading our immune system, breaking down our defense, promoting its growth and metastasis. No wonder cancer is often so challenging to achieve durable treatment success.
Many of the mechanisms mentioned are recent discoveries which have laid the foundation for new (often repurposed) therapies, not previously considered as candidates to treat cancer. Collectively, they highlight the importance of basic science to provide new insights for potential therapies. It takes years from such work to translate to effective, validated treatments, but hopefully illuminating this multi-dimensional hijacking capability of cancer will eventually enrich our armamentarium.
Thanks for reading and subscribing to Ground Truths.
If you found this interesting please share it!
That makes the work involved in putting these together especially worthwhile.
All content on Ground Truths—its newsletters, analyses, and podcasts, are free, open-access.
Paid subscriptions are voluntary and all proceeds from them go to support Scripps Research. They do allow for posting comments and questions, which I do my best to respond to. Many thanks to those who have contributed—they have greatly helped fund our summer internship programs for the past two years.


We need a cure, not endless "treatment". But a patient cured is a customer lost.