How Our Gut Microbiome Can Drive Sugar Cravings
More Evidence for Our Gut Taking Center Stage for What We Eat
A fascinating new report illuminated a pathway by which receptors in the lining of the gut, working through specific gut bacteria and a key metabolite, ultimately influence the brain’s sugar preference. In this issue of Ground Truths, I’ll review this elegant study and the gut-brain axis, which you’ll now see extending to the gut-liver—brain axis.
Parenthetically, Caity Weaver wrote the cover story in this week’s New York Times Magazine, a hilarious account of her trip to a medical spa in Austria to end her sugar addiction (here is a gift link to her article). A memorable quote: “What I had believed was my own preference was apparently the insatiable appetite of a foreign invader.” While that was mis-attributed to a “fungus that had hijacked my [her] mind in its relentless pursuit of sugar,” we now have evidence that a bacteria in our gut can play a role in our sugar cravings!

Before getting into the background, to let you know I’ll be doing a live zoom this week (Meeting ID: 978 1024 5216)
Join me for a live zoom conversation on Wednesday, January 29 at 10 AM PST
Here’s the link. We’ll discuss any biomedical topics of interest!
Background: The Gut-Brain Axis
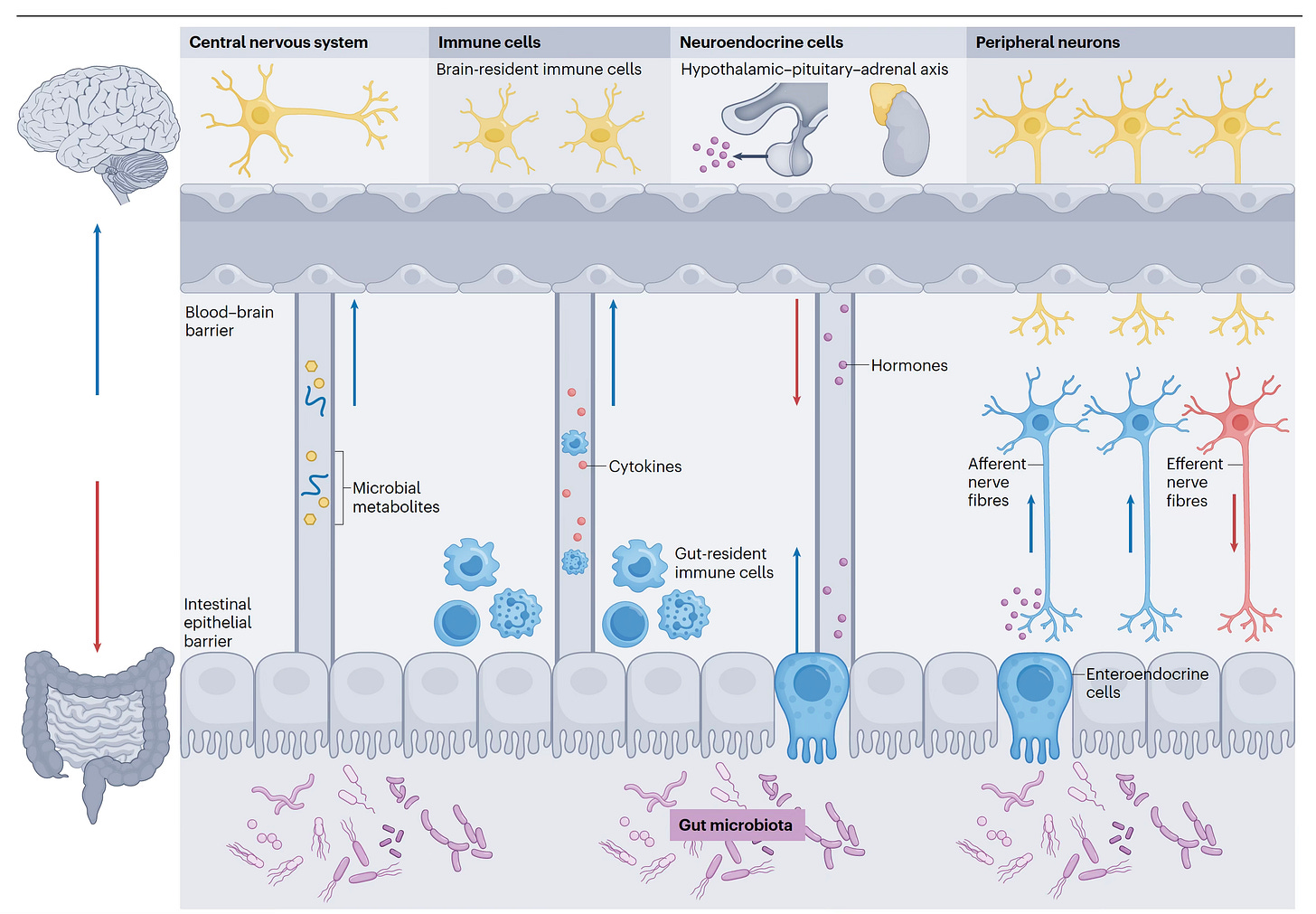
There are multiple ways the gut microbiome communicates with the brain, as seen in the Figure below and recently reviewed here. The 4 pathways are: (1) metabolites from the gut microbiome get into the bloodstream, cross the blood-brain-barrier, and affect the brain directly; (2) gut microbiome signals the gut-resident immune cells to either secrete cytokines and/or travel to the brain; (3) gut bacteria (or other microorganisms) trigger gut endocrine cells to release hormones, such as GLP-1, that enter the bloodstream and get to the brain; and (4) gut microbiota signals the gut (afferent) nerve fibers that communicate with the brain.
It’s not a one-way street. It’s reciprocal, a bi-directional relationship. That is, the brain also talks to the gut, regulating the gut microbiome, via the hypothalamus-pituitary axis and efferent nerve fibers through the vagus nerve plexus. Trying to keep it simple, there is extensive system (hormone, immune, neuron) crosstalk, such as between neurons and immune cells, and gut hormone producing cells and the immune system.
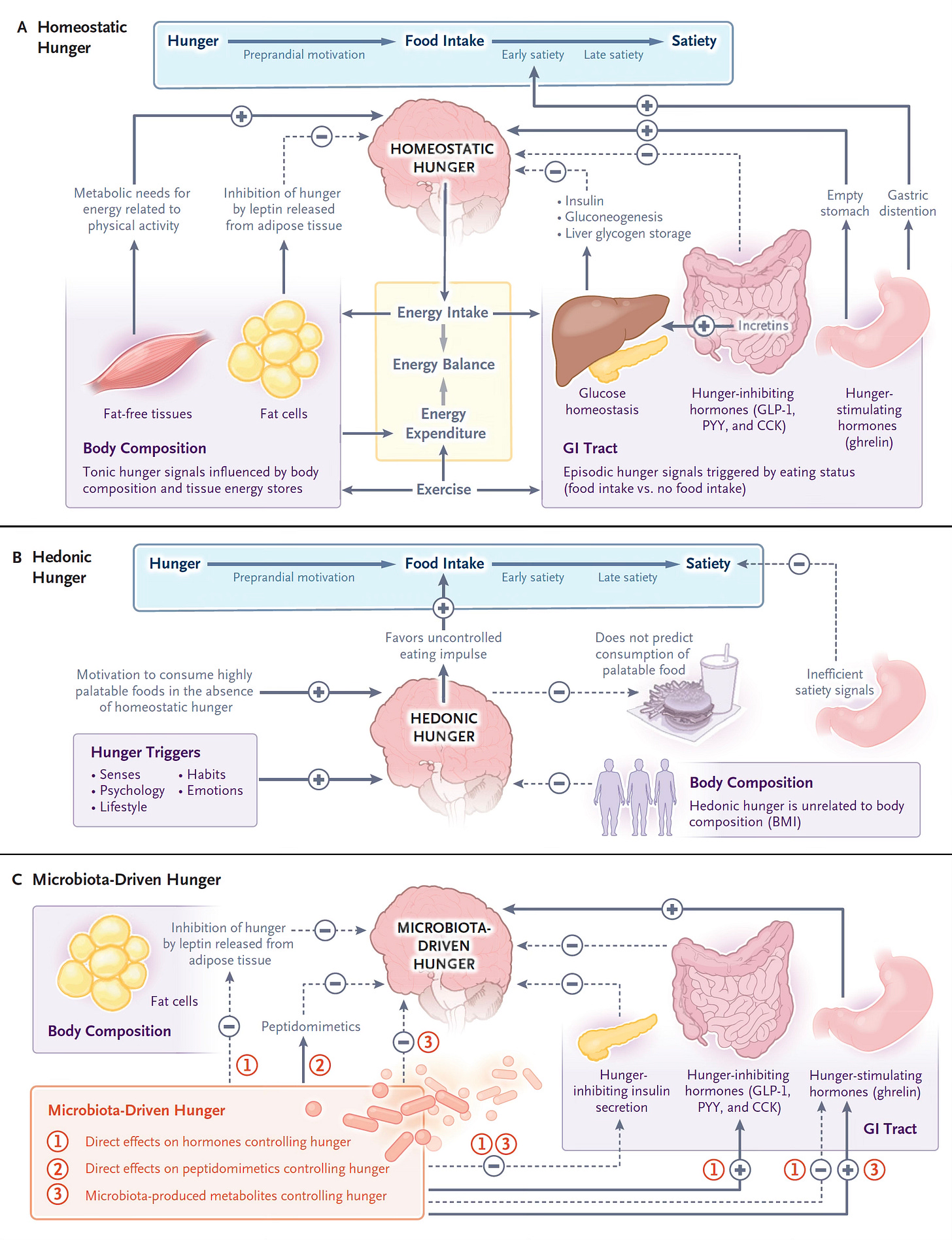
Background: One of the 3 Causes of Hunger is Gut Microbiome Driven
This week in the New England Journal of Medicine there is an excellent review article on the physiology of hunger. The 3 mechanisms that control hunger are shown below. The classic “Homeostasis Hunger” is driven by metabolic needs, whereas “Hedonic Hunger” describes food intake without homeostatic hunger but instead related to eating by impulsive, habits, stress and other stimuli. The 3rd mechanism (below, bottom) is the one related to the new report: “Microbiota-Driven Hunger.”
For example, indole, a metabolite of the gut microbiome, has been shown to suppress hunger by stimulating glucagon-like peptide-1 (GLP-1) and the gut microbiome makes Gamma-Aminobutyric acid (GABA) from dietary glutamate, which acts as neurotransmitter that communicates with the brain. People with obesity tend to have decreased glutamate producing organisms in their gut microbiome. These are just a couple of many more gut microbiome routes for driving or suppressing hunger that include gut hormone signaling, gut microbiome metabolites or protein products (called peptidomimetics).
The New Study
In the gut lining cells there are free fatty acid 4 (Ffar4) receptors. A systematic study of these receptors for their impact on the gut microbiome (in mouse models and people) shed new light on a pathway to sugar preference. When I say “systematic” I really mean it: this work involved a large array of experiments and analyses: 3 different mouse models of diabetes, with knockouts or over-expression of specific genes implicated, fecal transplants, a cocktail of antibiotic treatment, people with diabetes, Mendelian randomization (using gene alleles to establish causality), assessment of mutations of the Ffar4 gene in nearly 65,000 UK biobank participants and relationship with sweet intake preference, 16S ribosomal RNA gut microbiome sequencing, high-throughput metabolomics of gut microbiome constituents, administration of a GLP-1 drug (liraglutide), administration of liver-derived fibroblast growth factor FGF21 directly into the ventromedial hypothalamus (VMH) of the brain to nail down the direct effect, and more!
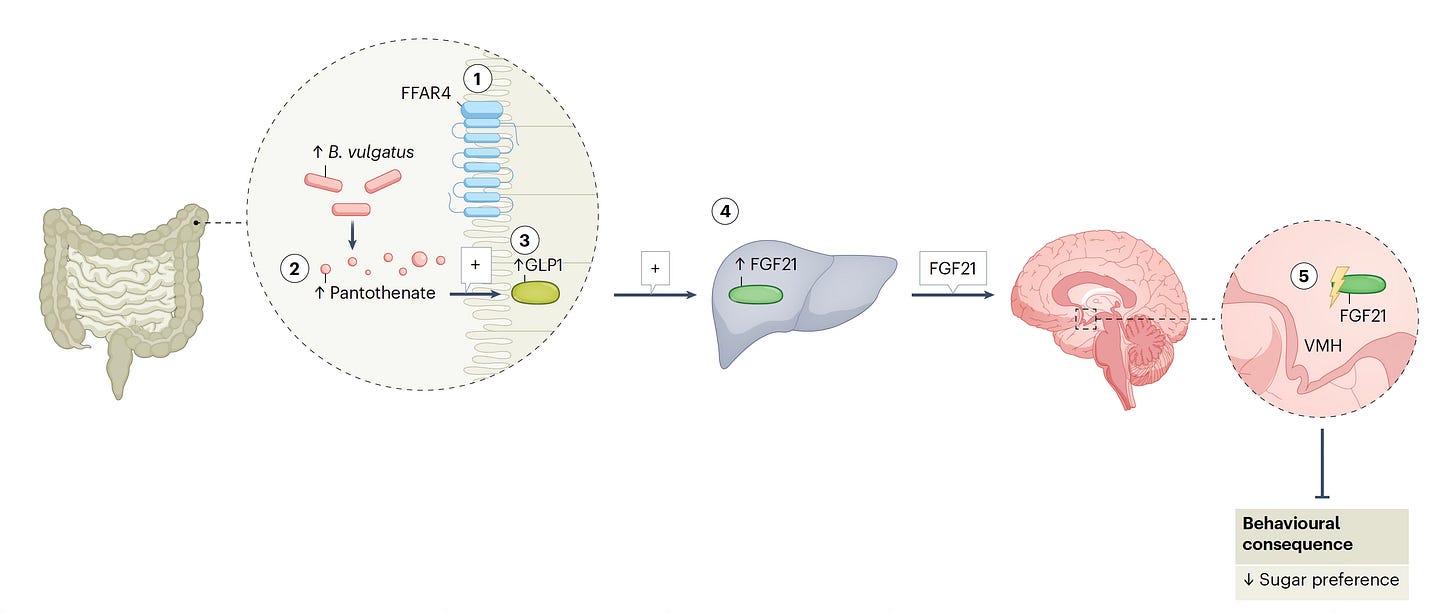
The authors provided a graphical summary of some the key experiments performed as shown below
Without going through each and every experiment, let me recapitulate the main findings:
In mouse models of diabetes and people with diabetes, FFar4 expression was decreased and this was linked to higher fasting blood glucose and sugar preference.
In a human population study (via UK Biobank) mutations in Ffar4, Mendelian randomization supported a cause and effect relationship of mutations and sugar preference.
Knockout of Ffar4 specifically in gut intestinal cells led to increased sugar preference, and over-expression of Ffar4 suppressed sugar intake.
The bacterium Bacteroides vulgatus was causally linked to intake of sugar. Levels of this bacterial species were higher in diabetic mice and humans and followed suit with Ffar4 knockout or over-expression for increased or reduced sugar preference, respectively.
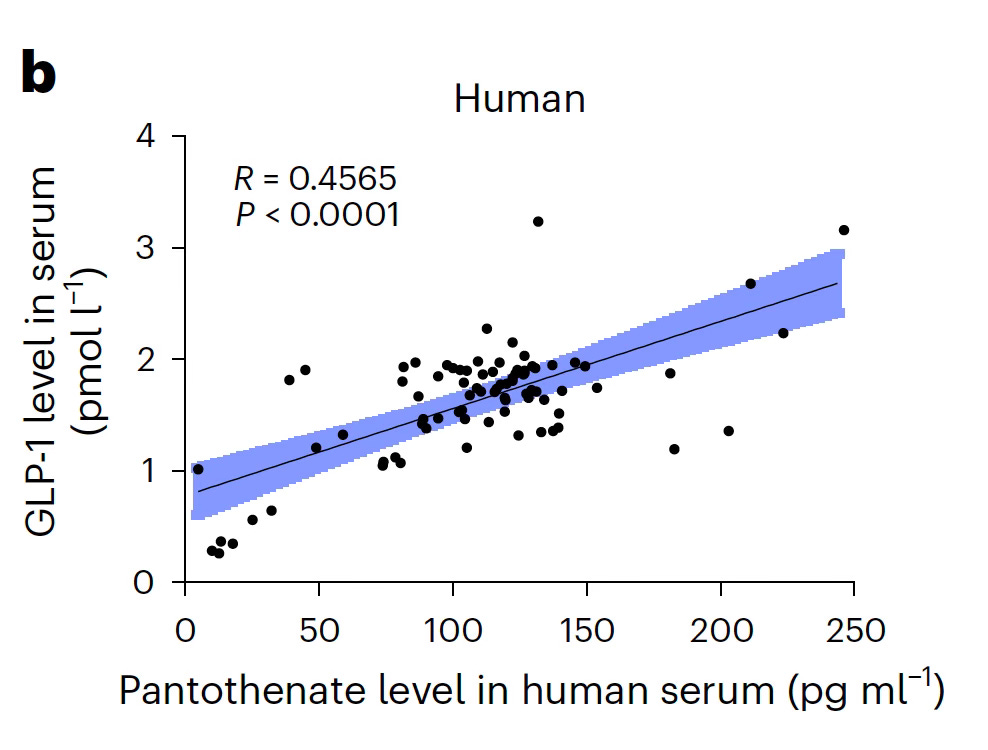
A key metabolite of B. vulgatus—Pantothenic acid (pantothenate, Vitamin B5)— was the metabolite ranking first of all metabolites significantly changed from B. vulgatus in culture, and repletion of it in the Ffar4 knockout mice suppressed sugar preference. This metabolite also increased GLP-1 levels in mice and humans (the latter shown in Figure below).
A GLP-1 drug (liraglutide) reduced sugar preference but when given directly into the mouse brain VMH region there was no impact on sugar preference.
GLP-1 stimulated the release of liver fibroblast growth factor 21 (FGF21). When FGF21 was directly administered to the VLM of the brain, it inhibited sugar preference. and FGF21 knockout or recombinant protein had the up or down effect on sugar preference.
In the accompanying commentary, a simplified graphic takes you through these various (5) steps for how Ffar4, working through the gut microbiome metabolite of B. vulgatus, with GLP-1 secretion and its stimulation of liver FGF21 then drives sugar craving via the brain’s VLM! Pretty remarkable stuff here.
Synthesis
This new report is extraordinary for pinpointing a specific bacterial species and its metabolite, and the chain of proteins (GLP-1, FGF21) that are secreted, for driving or inhibiting sugar cravings. Instead of “just” the gut-brain axis, we see the gut-liver-brain axis invoked, because GLP-1 alone did not affect sugar preference. The liver production of FGF21 was a requisite step. It is notable that the GLP-1 drug family (Ozempic, Wegovy, Mounjaro, Zepbound) is known to reduce a person’s food cravings, but the precise mechanism for that—be it directly through the brain (involving any of the 4 pathways in the first section of background above) or also working through the liver—has not yet been elucidated.
It’ll be important to replicate and extend the findings of the current report, including more clinical assessment and determining whether liver FGF21 is implicated in the GLP-1 drug effect of reducing food cravings, particularly involving sweets.
As I alluded to in the mechanism of hunger background, the current report is certainly not the first to implicate a gut microbiome species or its metabolite with food intake. We’ve seen it with Faecalibacterium prausnitzii and overeating and with Bacteroides uniformis with binge eating in experimental models. Beyond this, another recent study of a gut microbiome metabolite was linked to the process of aging. Surely the new Ffar4 paper indicates there will be many more like it forthcoming, featuring the gut microbiome, to help explain our eating preferences, cravings, and behavior.
There are many more implications from this work. We’ve long known that people with diabetes have a sweet tooth, and many potential explanations have been offered. Now there’s a new one. But beyond that, there is the potential of intervening to prevent or treat diabetes by gut microbiome manipulation (such as with pre- or probiotics) for B. vulgatus-derived pantothenic acid, or testing drugs (agonists) that would increase Ffar4 activity. Perhaps even a simpler strategy of supplementation with pantothenic acid (Vitamin B5) might be worthy of clinical trial assessment.
Getting back to the NYTimes Magazine piece, the study may have unraveled a basis for Caity Weaver’s sugar addiction (and one that many people share). Does she have Ffar4 gene mutations? Is B. vulgatus over-represented in her gut microbiome and what is the level of pantothenic acid in her blood?
Our food cravings are surprisingly getting unraveled. And they are also getting suppressed by the GLP-1 drug family, agonists of our gut hormones. Understanding the biologic underpinnings for these may someday take us— for explaining our food cravings— from “the devil made me do it” to saying it’s my gut microbiome metabolite X at work, and I’m going to fix it with my probiotic!
****************************
Thanks for reading and subscribing to Ground Truths.
If you found this interesting please share it!
That makes the work involved in putting these together especially worthwhile.
All content on Ground Truths—its newsletters, analyses, and podcasts, are free, open-access.
Paid subscriptions are voluntary and all proceeds from them go to support Scripps Research. They do allow for posting comments and questions, which I do my best to respond to. Many thanks to those who have contributed—they have greatly helped fund our summer internship programs for the past two years.
Please consider joining me for the live zoom this week (link at top of the post).




The Microbiomes have spoken: No guts, No glory.
This is a very interesting read, and validates what I have experienced, personally and with patients.
Personally, I have a sweet tooth. Twice I experienced having it evaporate: once it was after eating a GAPS diet for 6 days (!) -- the Gut and Psychology Diet, basically Russian grandmother diet. It starts very restrictive and then turns into a "heathy diet" full of produce and also meat over time. The effect lasted about 2 months and was very noticeable.
The other time was with a preparation of antimicrobial herbs. I don't believe in killing gut microbes willy-nilly and the Russian diet was too drastic for me but there must be other ways to budge that microbiome in the right direction.
I am launching my newsletter Tuesday: the first issue is on how Time-Restricted Eating (the practice of limiting your eating hours in the day) impacts your microbiome, and in turn, a variety of medical conditions.
I'm certainly linking to this newsletter in mine! https://simple-science.beehiiv.com/subscribe