How to misinterpret clinical outcome data in populations by age subgroups
The absolute importance of relative reduction
Recently a headline in the New York Times was “Younger People Benefit Less From Boosters Than Older”. Of course they do, as risk of nearly any intervention in medicine along with its absolute benefit are less with younger age. But to imply that boosters don’t help younger people is a flawed interpretation.
The Only Efficacy Trial of Booster Vaccination
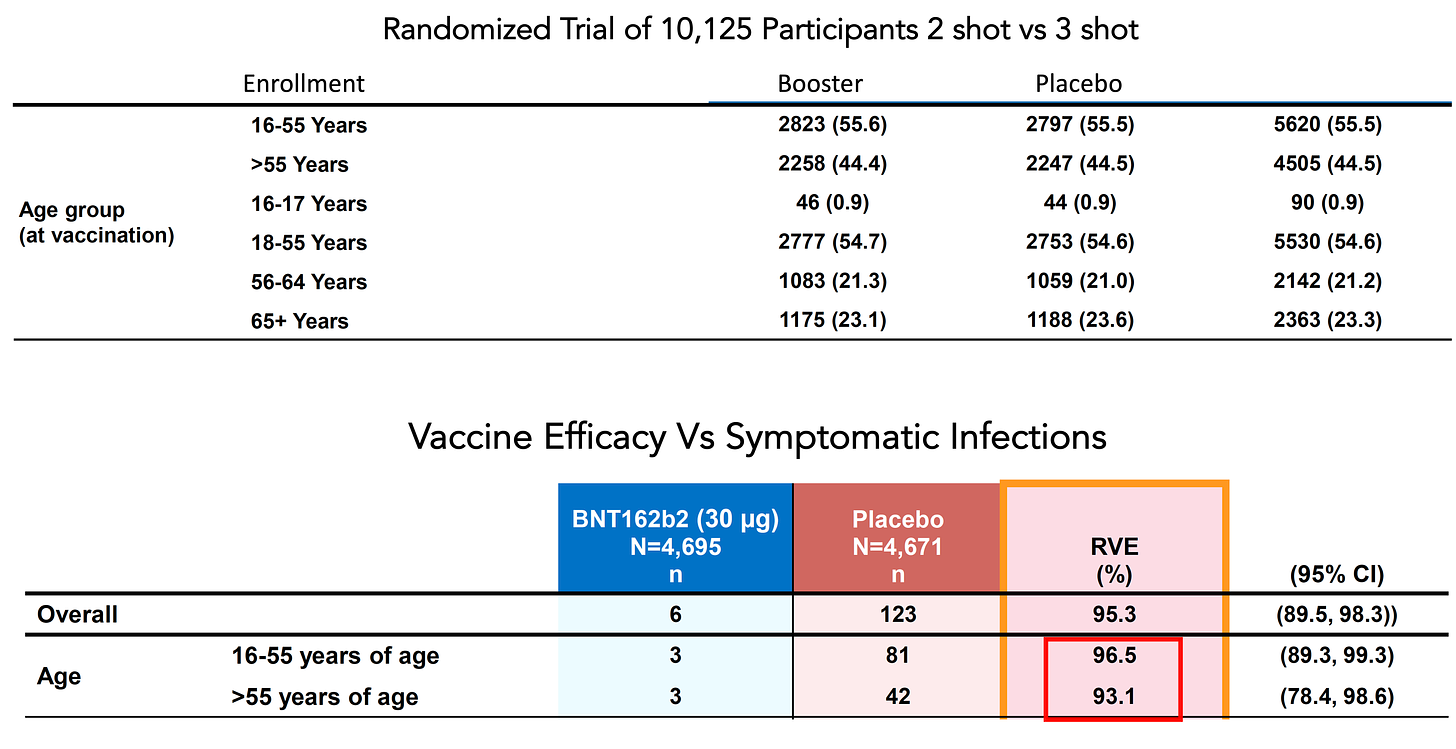
In fact, all of the data available for vaccinations and boosters in younger people indicates a highly consistent risk reduction as compared with those of advanced age. The only randomized trial of 2-shot vaccination + placebo vs 2 shots + booster in over 10,000 people demonstrated remarkable consistency for young vs older participants: a 96.5% efficacy for age 16 to 55 and 93.1% for age 55+ as shown below, with 55% of enrollees younger than age 55.
This trial was conducted during the Delta wave and the overall finding of a 95% efficacy for the booster was quite reassuring, since there was evidence from multiple reports that the risk of hospitalizations and deaths for this strain, and its higher level of transmissibility, were higher than the ancestral strain or prior variants of concern. Recall that the pivotal, placebo-controlled vaccine trials all used symptomatic infection as their primary endpoint, a proxy for the severe disease outcomes of hospitalizations and deaths. That was necessitated because the size of clinical trials to be adequately powered for reduction of hospitalizations and deaths would be prohibitively large and take far too much time.
This trial highlights why partitioning the results by age subgroups is problematic. Just the selection of the age cutoffs is arbitrary, even if pre-specified in a clinical trial data analysis plan. By definition, it looks at a particular group with inadequate statistical power for which a clinical trial could not be realistically done because of low absolute event rates—for the same reason we have not seen efficacy trials for hospitalizations and deaths. Does that mean vaccination doesn’t protect against hospitalizations and deaths? Of course not. We accept symptomatic infections as the surrogate and they consistently tracked with the severe Covid outcomes. For the same reason, we look to the older age people to get an estimate for what would happen if we could do large enough, adequately powered trials in the young. And we see in the case of the booster randomized trial an extraordinary level of consistency. We look for the direction and magnitude of effect, and whether it is consistent with the higher risk group. And indeed it is.
Vaccine Effectiveness Studies for Delta and Omicron Hospitalizations
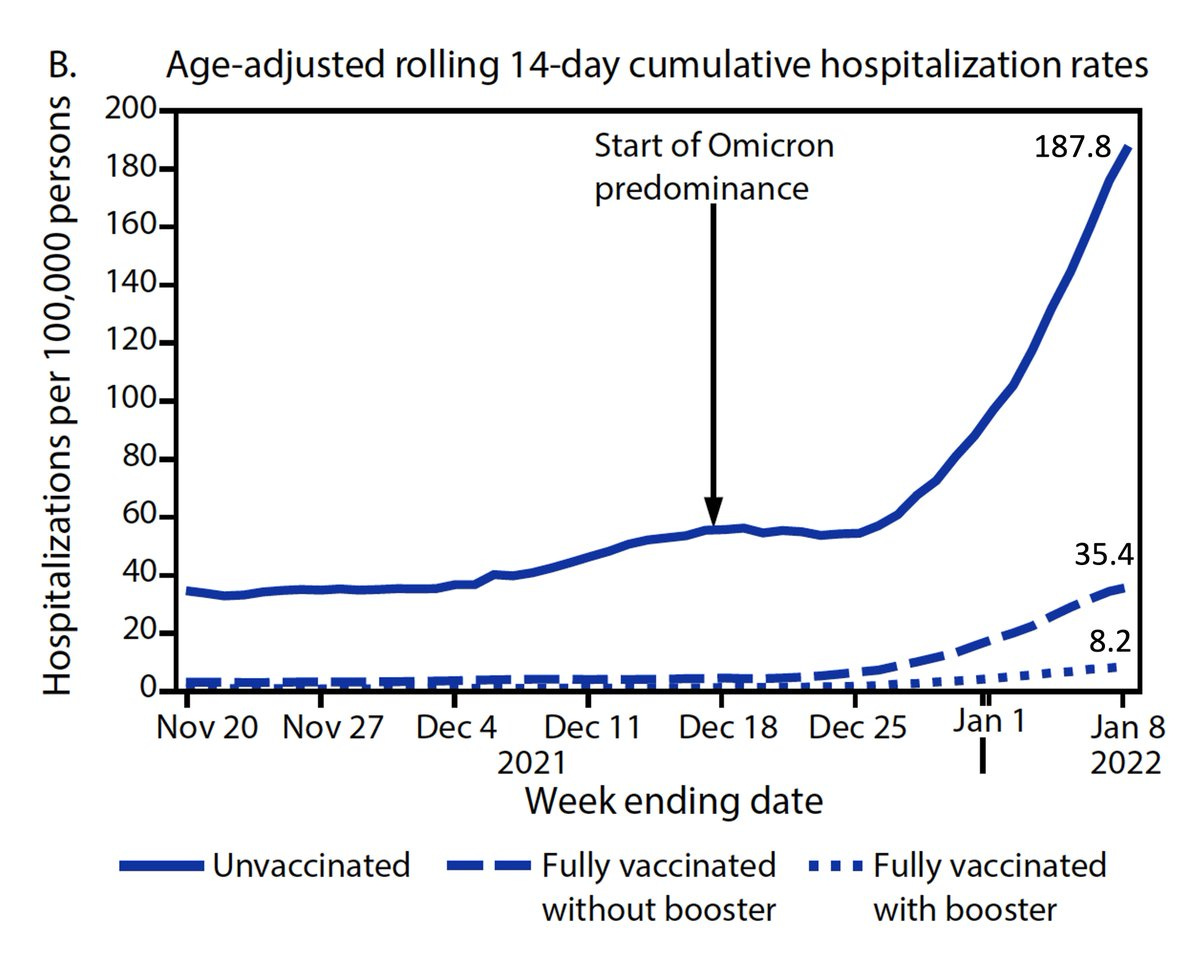
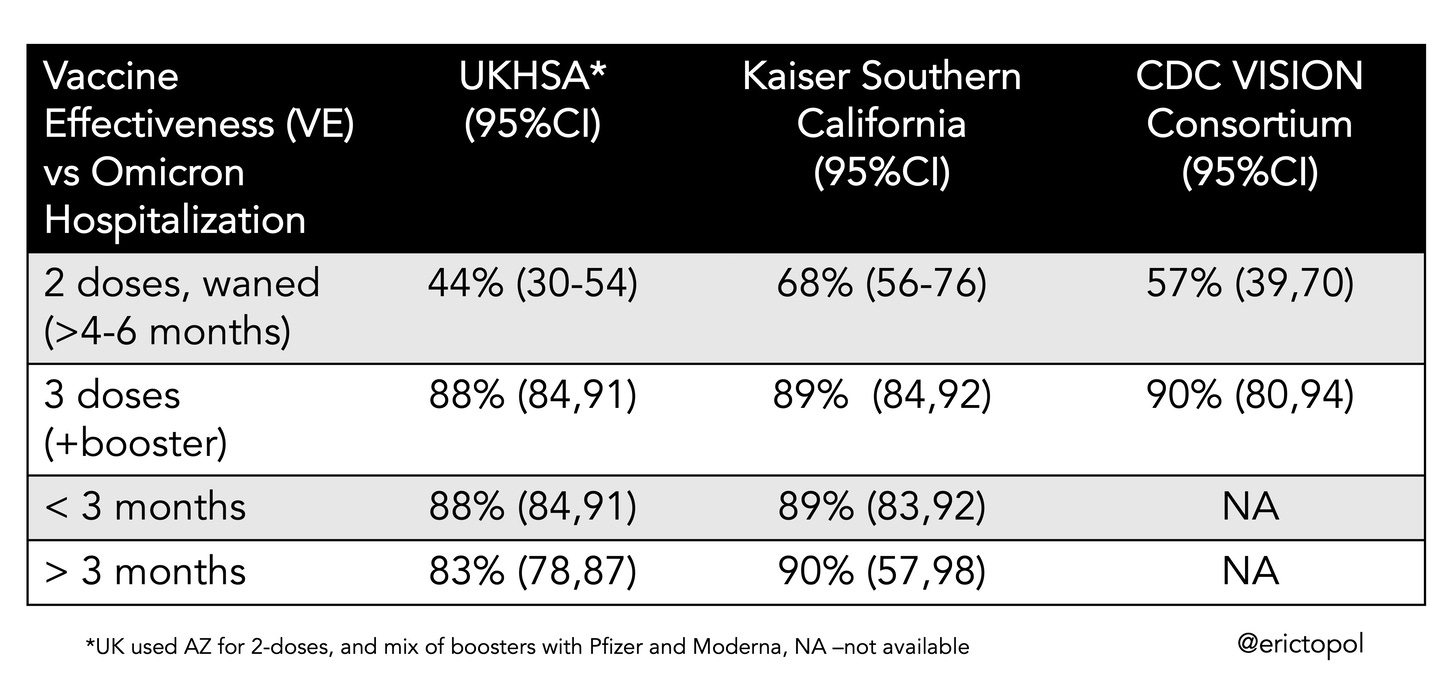
Now we transition to effectiveness studies in cohorts, without a placebo group, a reduced standard for evidence, but nonetheless quite meaningful. With Omicron, the issue of running low on statistical power is emphasized because of the immunity wall that has been built (by vaccinations and infections) and some intrinsic reduction in this strain’s virulence, with a resultant less incidence of severe disease. All of the datasets have the majority of people age less than 50 years. For example, the recent publication of the LA County data, the largest county in the United States with more than 10 million people, larger than countries such as Denmark, Ireland and Israel, showed an 81% reduction of hospitalization for 2-shots and 96% for 3-shots (vs unvaccinated) during the Omicron wave for age 18+. Does that mean it only applies to people over age 50? Of course not. The same applies for the 3 recent datasets from the UK, Kaiser Permanente Southern California and the CDC VISION network, as summarized in the Table below, all of which has more than half of their cohorts age less than 50.
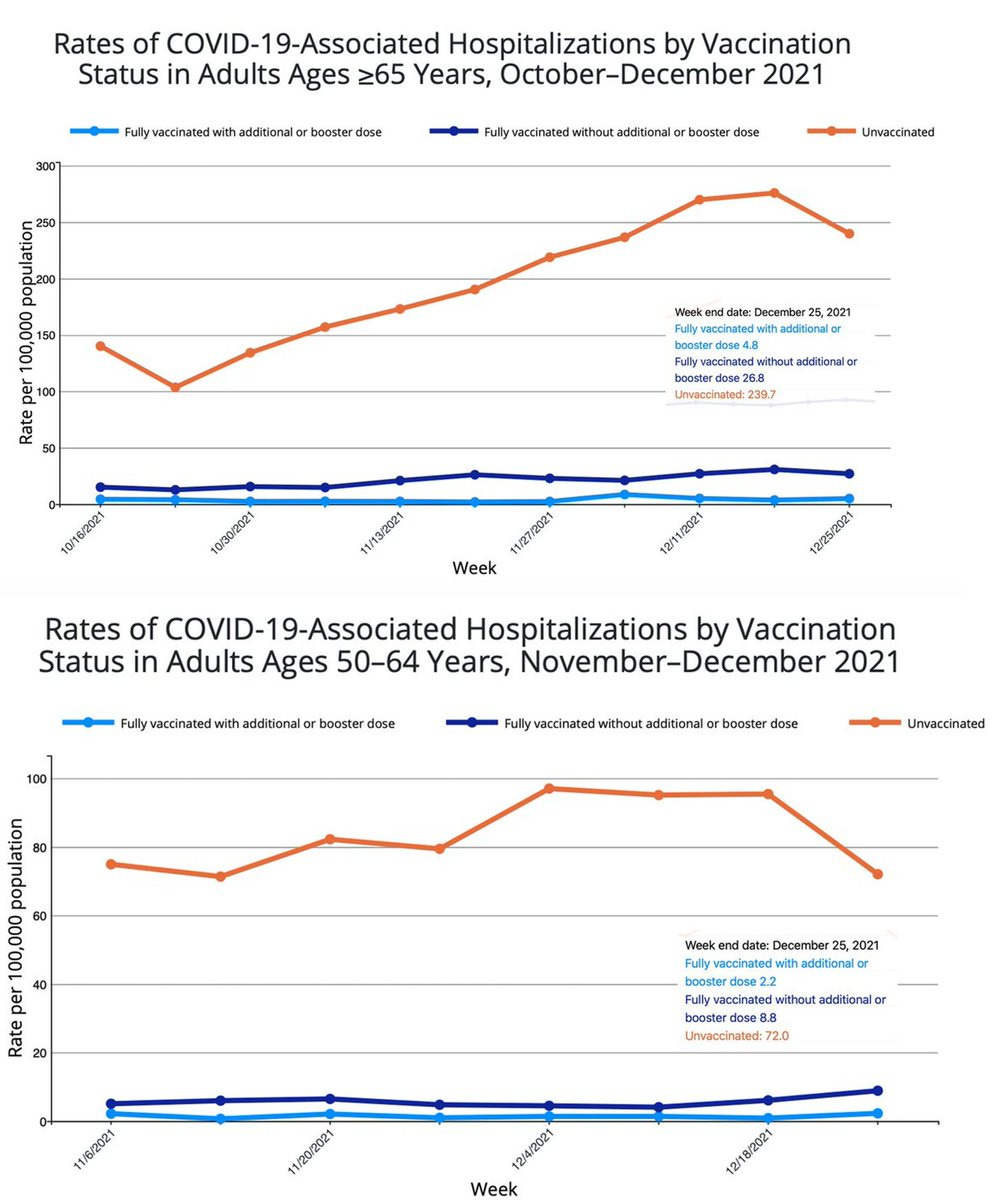
The CDC has not put data on their website for vaccination and booster effectiveness vs hospitalizations for age less than 50, but the consistency for relative reduction in the 2 groups here, age 50-64 and 65+ for boosters is remarkable: 97% and 98%, respectively. There is no reason to believe that relative reduction would not also be present in younger age groups and there are multiple individual states and countries which confirm that point.
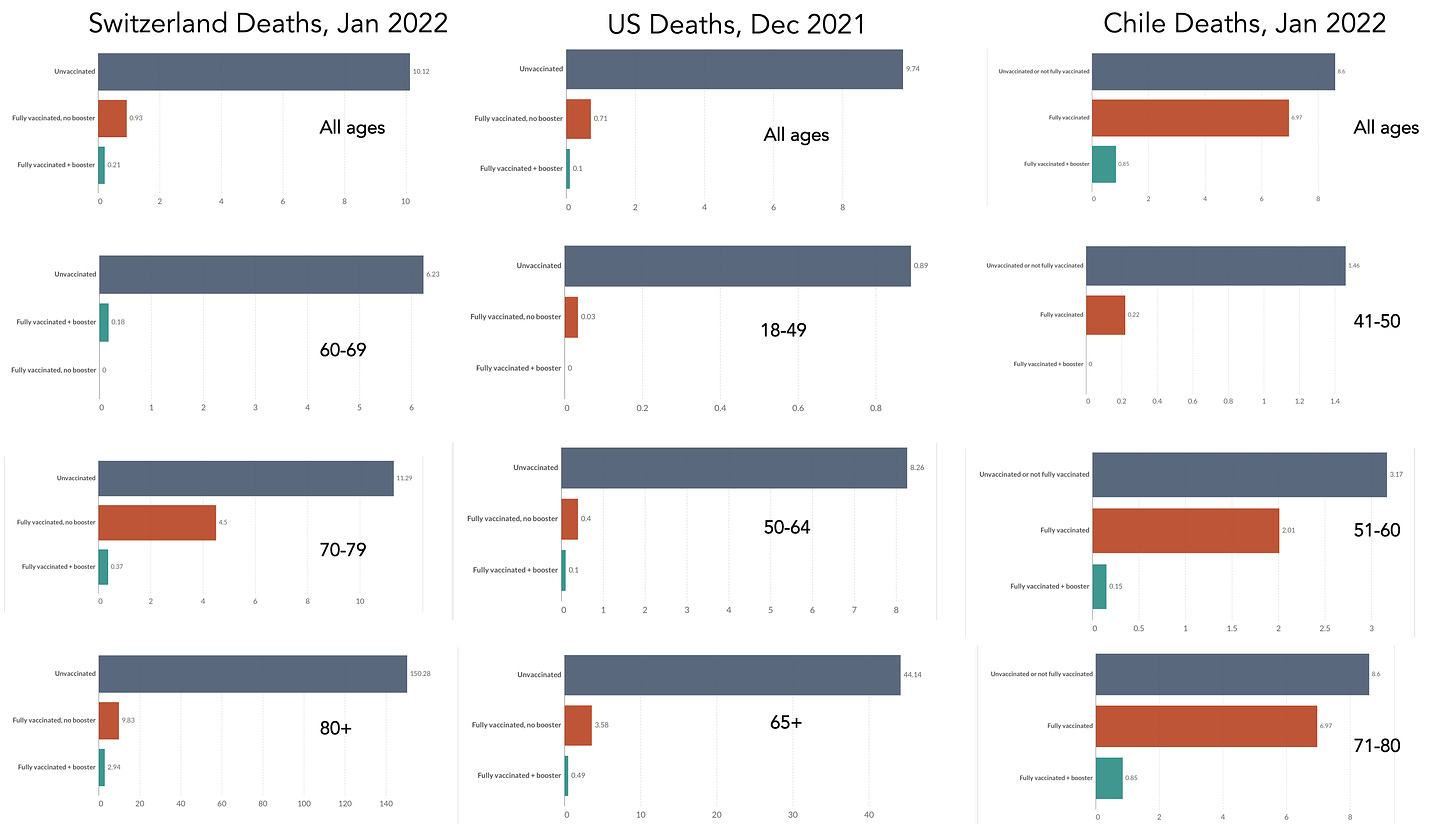
Vaccine Effectiveness Studies for Delta and Omicron Deaths
Here are recent data for 2 and 3-shot vaccinations vs deaths across all ages and for some age subgroups for Switzerland, the United States, and Chile. The data from Chile are a bit more difficult to compare with >75% use of Sinovac vaccines, which have been shown t exhibit lower effect vs Omicron. In both Switzerland the Chile there are no deaths among vaccinees younger than age 70 and 40, respectively. In the United States across the 3 subgroups, there was a consistent 99% reduction of death for vaccination + booster vs unvaccinated. As you look across all the subgroups, you see the consistency that the booster group has the lowest mortality in each country for each age group. As more countries provide such data, this will further prove the point that relative reduction of deaths is consistent across all ages.
When it comes to looking as subgroups, it is important to focus on directionality, consistency, and magnitude. No matter how we look at the booster vaccine data, for Delta or Omicron, for hospitalizations or deaths, for efficacy or effectiveness, there is one message. Young people benefit as much as older on a relative basis, which has fully justified the policy of administering booster doses for all ages 16 or 18+ in countries throughout the world. Any statement asserting lack of benefit in the young fails to acknowledge the central interpretative caveats of subgroup analysis and the primacy of relative risk reduction.