Inching closer towards a variant-proof, pan- coronavirus vaccine
Guest Post with Dr. Jetelina
We teamed up to review the implications and progress towards a vaccine that would be effective against the beta-coronavirus family, including all variants of SARS-CoV-2
Why would this be needed?
While our current vaccines continue to work swimmingly well against severe disease or death, it’s become increasingly clear that the current vaccines have limitations. The neutralizing antibody response (i.e. first line of defense) mounted by mRNA vaccines wanes after a few months, and we’ve seen a decline in vaccine effectiveness against symptomatic infections to go along with that. As a result, Israel is rolling out a 4th vaccine for those aged 60+ years. It’s also clear that this virus will continue to evolve with mutations that are hard to predict. While Moderna and Pfizer have announced their plan to make Omicron-specific vaccines, we will ultimately need to transcend a Greek letter approach with a vaccine that would be effective for the entire coronavirus family, essentially making it variant-proof.
An ideal solution is a universal coronavirus vaccine that would not only protect against SARS-CoV-2 but also against other coronaviruses that might cause future animal outbreaks and pandemics. Scientists have been advocating for this type of coronavirus vaccine since as early as May 2020 and as recently as the beginning of this month.
We’ve been working for years on a pan-vaccine for other viruses, like HIV-1, influenza, malaria, Epstein-Barr (NCT03186781; NCT03814720; NCT04579250; NCT04645147; NCT04296279), but no vaccine has successfully made it through clinical trials thus far. The difference for SARS-CoV-2 is that it mutates far less than HIV or influenza, making it more ideally suited for such an approach. If any universal vaccine makes it through the rigorous clinical trial process, it will be a big leap in medicine and science.
How does the “super” vaccine work?
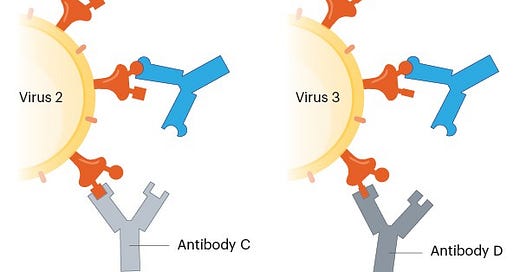
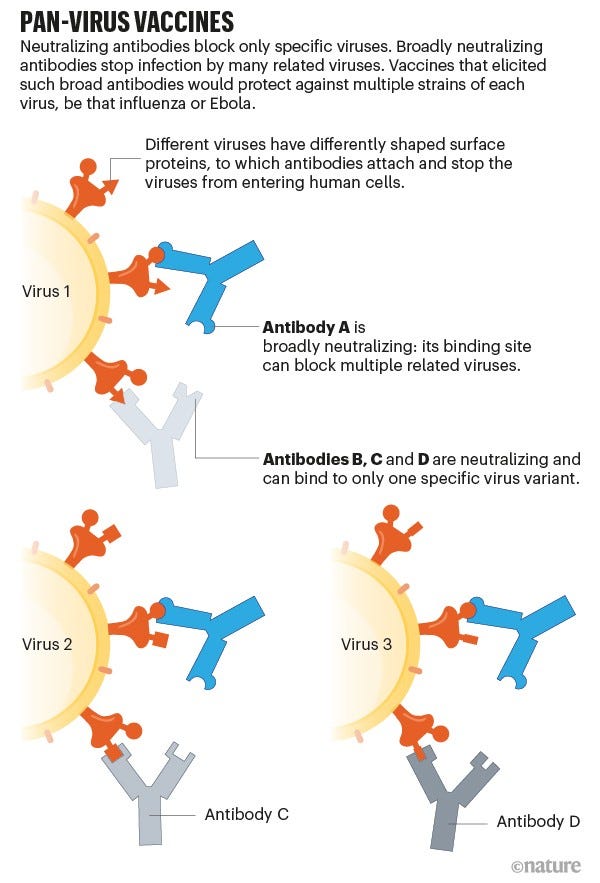
The main approach taken by research labs is to find people called “elite neutralizers” who have had COVID19 or been vaccinated (or both) but have an incredibly rare and unique response. These are people who can make very potent antibodies (called broad neutralizing antibodies; bnAbs) that bind to parts of the virus in the spike protein—parts of the virus that are conserved, that is present in all of the coronavirus family (potentially beyond the spike protein), remain unchanged despite mutations, and are hidden (“cryptic” epitope sites). These elite neutralizer people are rare, but there’s basically a treasure chest of protection lying within them.
More than 10 scientific groups have found such potent bnAbs. For example, scientists at Duke compared antibodies among a SARS patient and compared it to antibodies among a COVID19 patient. They looked at 1,700 different antibodies in total and found 50 antibodies that were able to bind to many different coronaviruses. The Duke researchers then worked with the UNC-Chapel Hill to test whether these antibodies effectively blocked infections in mice. This science was published in early November and found that these antibodies were very effective. But finding these “elite neutralizers” is half the battle. Then a reverse engineering approach is required to make vaccines that produce the bnAb. After that, the vaccine goes into pre-clinical and clinical trial testing.
A faster approach
A faster approach is the one just published by the Walter Reed Army Institute of Research using “nanoparticle vaccine technology.” The vaccine presents a protein that looks like a soccer ball with many different faces (see figure below). Each face presents instructions for a different part or version of a virus. Then our body makes antibodies for each one of these faces and ensures that our antibody factories (called B-cells) remember these different designs.
The tough question that scientists are trying to answer is: what should they include on these faces? Walter Reed is testing two other approaches that focus just on the spike protein:
Spike Ferritin Nanoparticle (SpFN) COVID-19: This vaccine looks like a soccer ball that has 24 different faces. Each face carries a different spike protein drawn from coronavirus variants.
Spike Receptor-Binding Domain Ferritin Nanoparticle (RFN): This vaccine looks essentially like #1 but targets a much smaller part of the coronavirus spike protein called the RBD. This science was just published in the National Academies of Science proceedings here.
Pre-clinical trial
Walter Reed’s first approach above (24 faces of the spike protein) made a splash in the news this week after scientists published their pre-clinical trial results. The pre-clinical phase is the first step in clinical trials: How does this vaccine protect non-human primates (monkeys) from disease?
To answer this question, scientists gave the new vaccine to 32 monkeys. Animals were vaccinated with one of two doses: 50 or 5 μg. Then they were given the same dose 4 weeks later. The animals were then challenged with several COVID19 variants (like Alpha and Beta) and the SARS-CoV-1 virus that emerged in 2002. Omicron was nottested in this phase because it wasn’t around at the time. What did they find?
Both doses induced neutralizing antibodies (first line of defense) against all variants and coronaviruses tested
The high-dose (50 μg) induced a T-cell response (second line of defense)

Other scientist groups at Walter Reed tested this vaccine against mice and another group among hamsters. These studies were published earlier this month (here, here) and found the same thing: The vaccine works among animals.
Phase I trial
Because the pre-clinical phase was a success, Walter Reed started Phase I earlier this year (April 2021). A total of 72 healthy adult human participants (age range 18-55) were randomly assigned a placebo, 25 µg vaccine, or 50 µg vaccine. Each group received their injection on Day 1, Day 29, and Day 181 (three-dose series). The scientists are also testing 50 µg of the vaccine at Day 1 and Day 181 (two-dose series). This clinical trial has taken longer than expected because people can only participate if they have not been previously vaccinated or infected with COVID. As you can imagine, the pool of eligible and willing participants is getting smaller and smaller.
Nonetheless, after receiving the trial vaccine, participants’ blood samples are tested against variants of concern, including Omicron, and other types of coronaviruses. Results will provide two critical pieces of information:
How well does the vaccine carry over from animals to humans?
What is the safety profile of the vaccine? What are the side effects?
We’re waiting for the results to be released, which should happen soon. Assuming Phase I proves to be safe and effective, the new vaccine will undergo Phase II and Phase III trials. It will be awhile before we know more about its safety and effectiveness, but this science reflects remarkable progress toward having a variant-proof vaccine.
Bottom line: This vaccine is the first candidate for pan-coronavirus and is way ahead of others because it has already passed through non-human primates and Phase I human volunteers. This pandemic continues to prompt very exciting, innovative research. If found successful, our fight against COVID19 and coronaviruses beyond will be forever changed.
Love, YLE and Eric Topol
I was lucky enough to collaborate with Eric Topol on this post— scientist, professor of Molecular Medicine, and Founder and Director of The Scripps Research Institute. He’s published on pan-coronaviruses and helped me think through what these vaccines do and their exciting potential.
“Your Local Epidemiologist (YLE)” is written by Dr. Katelyn Jetelina, MPH PhD—an epidemiologist, biostatistician, professor, researcher, wife, and mom of two little girls. During the day she has a research lab and teaches graduate-level courses, but at night she writes this newsletter. Her main goal is to “translate” the ever-evolving public health science so that people will be well-equipped to make evidence-based decisions, rather than decisions based in fear. This newsletter is free thanks to the generous support of fellow YLE community members. To support the effort, please subscribe here: