The Big Trial of a GLP-1 drug (Wegovy) to Reduce Major Cardiovascular Events
Presented at the American Heart Association and simultaneously published in NEJM today is the trial of over 17,600 patients who were randomly assigned either the GLP-1 semaglutide (a glucagon-like peptide-1 receptor agonist) or placebo and were followed for well over 3 years.
We have eagerly awaited seeing the actual results since the sponsor company (Novo Nordisk) issued a press release in August that proclaimed a 20% relative reduction of cardiovascular death, heart attack or stroke, the primary endpoint of the trial.
The trial participants were at very high risk for subsequent cardiovascular events with 68% having had a heart attack (82% had coronary artery disease) and the rest with prior stroke or peripheral arterial disease or a combination of these entry criteria. Even though a BMI >27 kg/m2 was required for enrollment, the mean BMI of enrollees was >33 kg/m2; over 70% had baseline criteria that met obesity. There was a lack of diversity with 84% White, <4% Black. Participants were not diabetic at baseline, but two-thirds had a HbA1c at or above 5.7% which is abnormal, often referred to as prediabetic. The average age at entry was 62 (age 45 plus was an entry criteria). The average LDL at entry was 78 mg/dl which is not optimal in such a high-risk population.
The trial was set up to detect a 17% reduction in the primary endpoint with 90% power. There was 1 interim analysis in July 2022 with 831 events that led to continuation of the trial to its end, with a minimum of 1225 events—the trial ended with 1270 total events exceeding that objective.
The good major findings
Semaglutide did reduce major events in this high-risk population by 20% and would be considered the first drug directed at obesity to have achieved this goal.
The 40 month follow-up is the longest thus far for a large trial of semaglutide vs placebo, which gives some confirmation of safety for this duration of therapy.
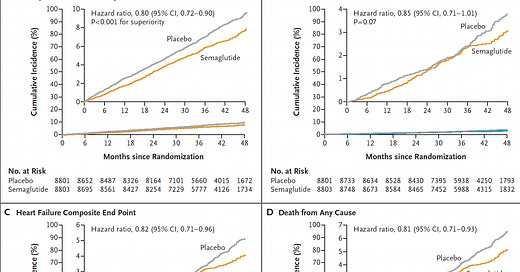
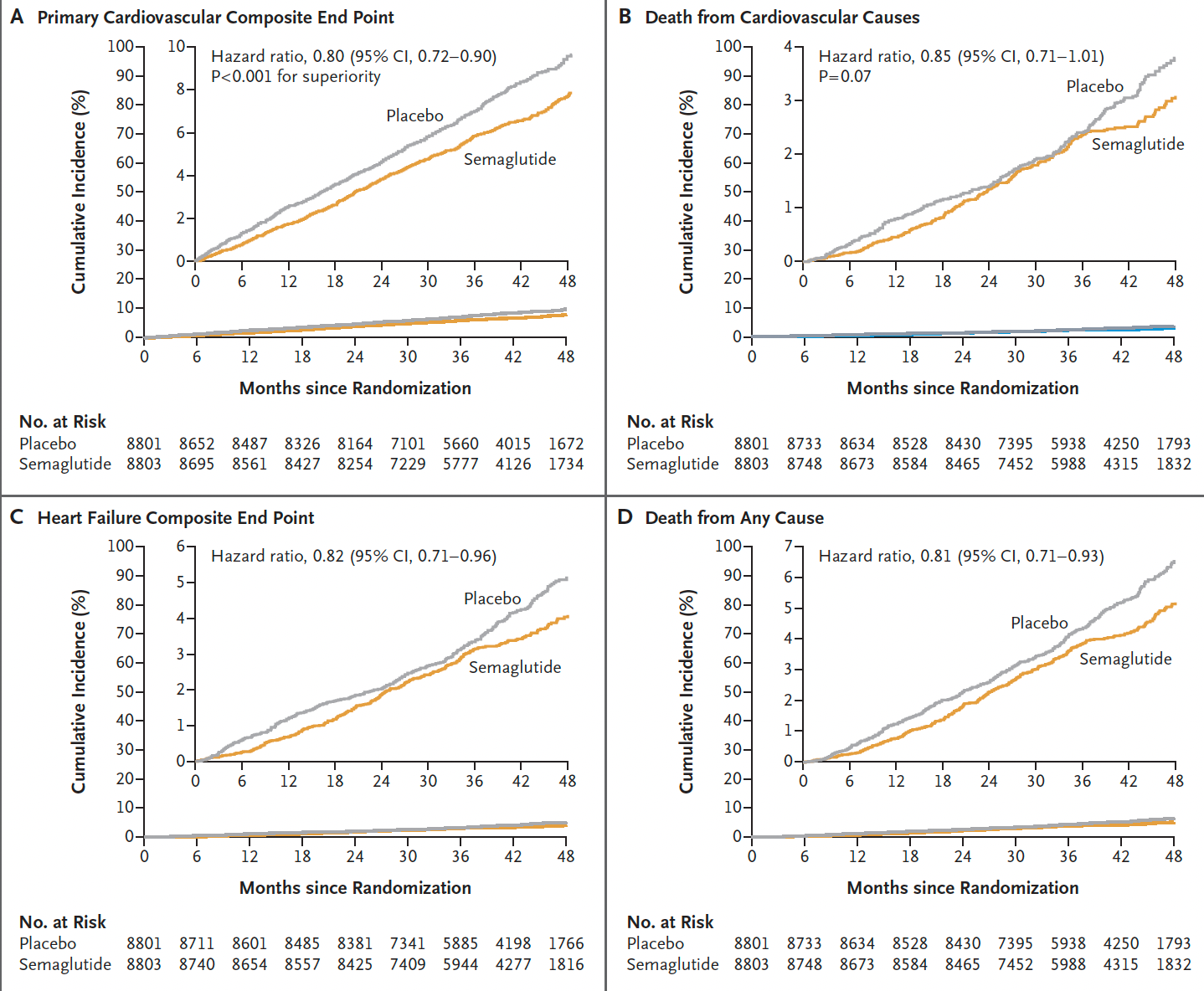
There was a fairly consistent ~20% reduction across the different endpoints, although the time seen for divergence of the cardiovascular death curves or death from any cause (graphs below) was delayed > 3 years, in comparison to early separation of the curves for non-fatal endpoints (mainly heart attack reduction).
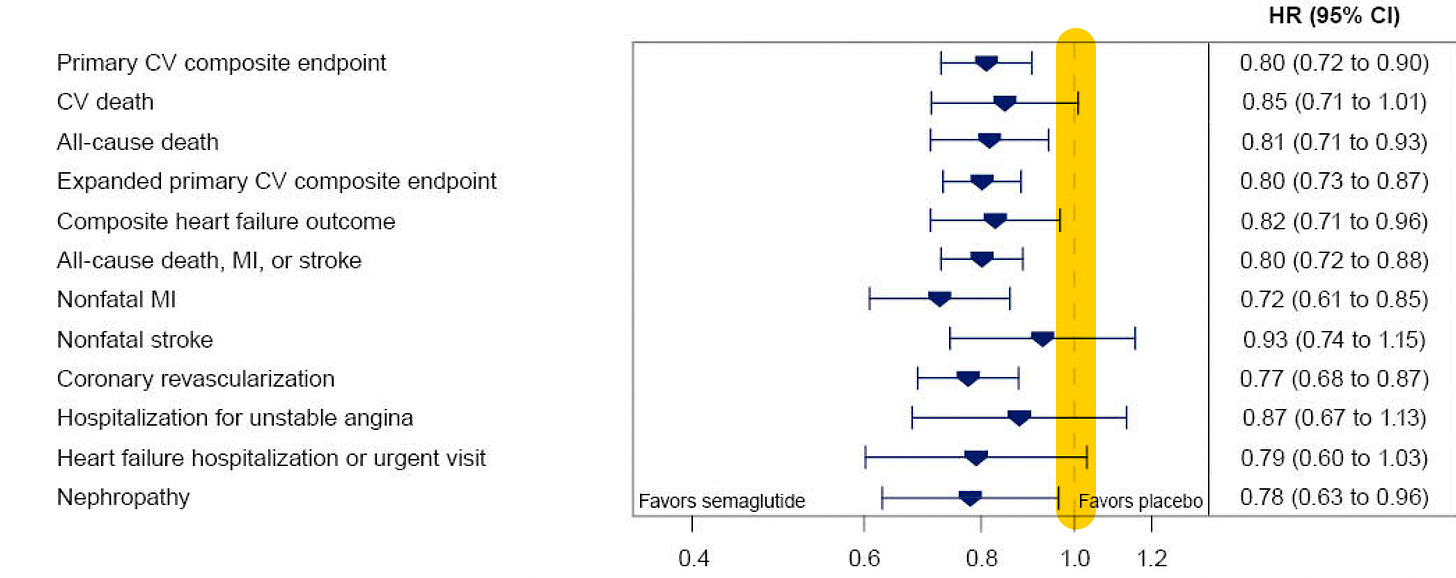
Here is the time-to-event analysis which again shows the non-fatal heart attack (MI) reduction, whereas nonfatal stroke was not significantly reduced (a 7% relative reduction). The yellow vertical line is the line of identity (1.0 hazard ratio)—if the 95% confidence intervals crosses it, that specific outcome is not statistically significant.
As might be expected, the reduction of progression to diabetes was striking: only 3.5% progressed to HbA1c > 6.5% in the semaglutide group as compared with 12% in the placebo group, a 73% reduction. Along with this there was a fairly pronounced reduction of the C-reactive protein inflammatory marker 39% vs 3% reduced, for semaglutide vs placebo, respectively.
The findings were independent of overweight/obesity category at baseline, as seen in this subgroup analysis for the primary endpoint. In fact, the subgroups < 30 and 30-35 BMI demonstrated the most relative reduction.
Not so good findings
The absolute reduction of the primary endpoint is only 1.5 per 100 people treated, with the people in the trial representing a very high-risk cohort. And they had to be treated for 3+ years to derive that small absolute benefit.
Note that the price of Wegovy in the United States is $1,349 per month.
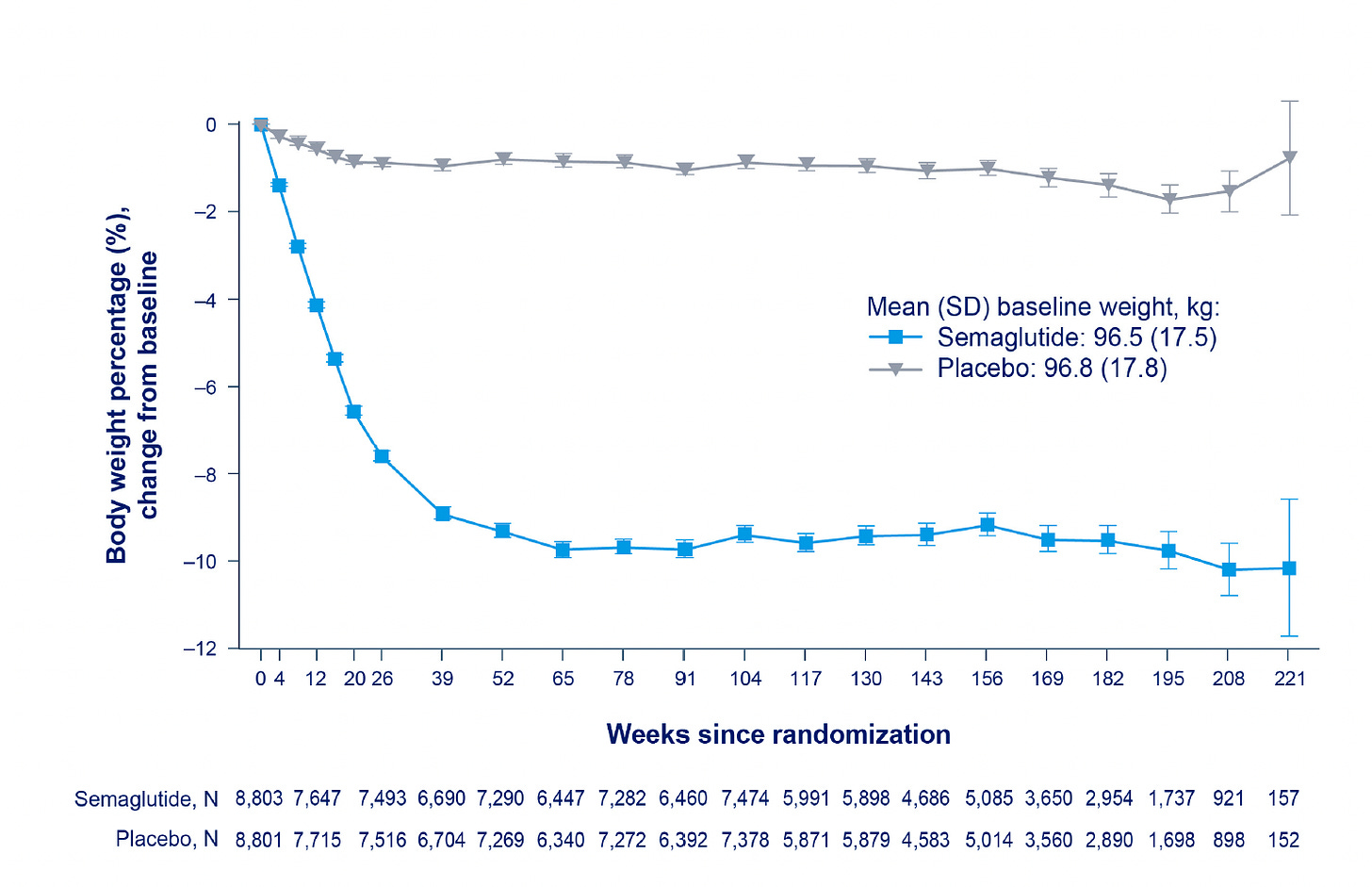
The body weight loss achieved was only 8.5%. As I reviewed last December, this is considerably less than was seen in the Wegovy and Mounjaro previous, smaller randomized trials with less follow-up time (<1.5 years). It remains to be seen whether more extensive weight loss would further reduce cardiovascular events, and to what degree was the weight loss per se affecting the outcome improvement or driven in part by other effects of the drug, such as reduction of inflammation.
Prior GLP-1 randomized trials for weight loss reduction
There is no exit strategy. Beyond losing weight, we know that people on these drugs lose muscle mass (not measured in the trial) and bone density, and the companies marketing these drugs have done nothing to assess strategies of weaning and discontinuation of the drugs to avoid these untoward effects, no less others that may crop up after extended (multi-year) exposure.
Summary
This well conducted GLP-1 drug randomized trial is important for documenting reduction of cardiovascular events in a high-risk cohort. But the absolute reduction (1.5 per 100 treated for >3 years) is quite low, especially given the high risk of the population and the high cost of the drug. Paying out of pocket for the full price of $1,349 per month for 36 months equates to >$48,500. It is likely that insurers, and Medicare, will revise their coverage on the basis of the trial to cover the drug for high-risk patients that fit the entry criteria of the trial, but that will represent a very large economic burden to cover the drug cost. The FDA approval of Zepbound (tirzepatide, same as Mounjaro but given another name) this week with a cost of $1,060, is a bit better. Access to these drugs is likely to exacerbate inequities, added on to the lack of diversity of the people assessed in the trial.
Much more work is needed to resolve the question of GLP-1 benefit for people who are obese but without prior heart attacks or at high risk for cardiovascular events—we know nothing about that yet. It is possible that more potent GLP-1 drugs, and triple-receptor agonists will achieve a greater degree of weight loss and extend the benefit to lower risk people in the future. All that remains to be seen, and an absolute benefit of 1.5 per 100 people treated is certainly nothing to write home about.
To me the biggest concern is that these companies appear to be promoting a lifelong duration of therapy, as evident by their zero attention to getting people off the drugs, without reverting back for both weight gain and risk of adverse outcomes. That is clearly unacceptable and major pressure is needed to get all companies making this class of drugs to test and validate durable and safe exit strategies.
Thanks for reading and subscribing to Ground Truths!
Please share if you found the post informative.



Thank you so much for your clear, fair description and assessment of this study. Reading this together with your post on ultra processed foods offers a powerful demonstration of the skewing of our health care system toward profit-making over seeking the most beneficial pathways to better health. Your final point brings this home: “To me the biggest concern is that these companies appear to be promoting a lifelong duration of therapy, as evident by their zero attention to getting people off the drugs, without reverting back for both weight gain and risk of adverse outcomes. That is clearly unacceptable and major pressure is needed to get all companies making this class of drugs to test and validate durable and safe exit strategies.”
In the U.S., the obesity epidemic is the consequence of the corporate food epidemic. The "invisible hand" of unregulated free markd capitalism ceated both the fast food resturarant epidemic, which is built on shifting our diets to addict us to our hunter-gatherer food cravings - saturated fats, super-glycemic carbs, and the modern Ameriican supermarket, which profits from ultra-processed foods, emerging from the labs of large corporations ,which create easily-prepared, food-like toxins, where "new and improved" meals, means more sugar, more fat , more "mystery" ingredients, often added to replace nutritious ingredients that have been removed. Forget the fact that to get to a fast food restuarant, or supermarket, by any means other than sitting in a car is often either impossible, or dangerous. Scientists do not understand the multiple, extraordinarily complex, physiology and pathophysiology of nutrition, nor the consequences of not exerting our bodies regularly, which we evolved to do as well. Mediciations can do many wonderful things. but Medications cannot "fix" physiological systems that we do not understand - medications can reduce the damage we self-inflict. As physicians, if we are to claim to be evidence-based, we need to speak truth to power - currently the evidence base indicates we are grossly neglecting the causes of the diseases - our food, and our designed environments, which are built to maximize proift, regardless of the fact that they destroy our heatlth.