Why Paxlovid is a Just-in-Time Breakthrough
The first potent anti-Covid pill will be a big help
We’re desperately in need of some good news—this is it. This week the results from 2 clinical trials of Paxlovid (nirmatrelvir) became available, coinciding with the continued (second) surge of Delta in the United States and the rapid emergence of the Omicron strain. The timing for validation of the potency and safety of a whole new approach to tackling the virus couldn’t be better.
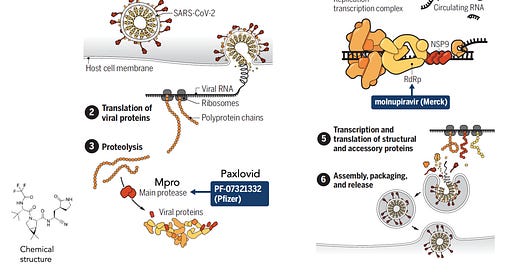
First let’s review the mechanism of action. Paxlovid, a small molecule that is orally active, was specifically designed to tackle SARS-CoV-2. It is not a repurposed drug, but actually derived from a precursor that was active against SARS. It binds to the virus’s main protease (Mpro) as shown here, a key upstream step before it gets into high gear replication (steps 4-6). Inhibiting Mpro is a choke point for the virus, making it unable to replicate.
Why is this so important?
Until now in the pandemic, our prevention and early treatment of Covid has relied upon our immune system: vaccines and monoclonal antibodies. Our immune response, at least its humoral component— antibodies—has been substantially evaded by Omicron. This new strain has put a major dent into the effectiveness of vaccines to prevent symptomatic infections, even with a third shot (approximate effectiveness of 75%, reduced from 95%, correlating with an ~5-fold increased risk of breakthrough infections). And that level of protection may decline further over time, the extent to which has not yet been established. Moreover, the monoclonal antibodies and their combinations that potently and quickly inactivate prior strains of SARS-CoV-2 have now been shown to lack that effect for Omicron (Table), with the possible exception of Sotrovimab, as was just independently replicated. So right now we desperately need a strategy that is not dependent on our antibody neutralization response.

Along Comes Paxlovid
A pill that could be readily administered which does not rely on our immune response. Omicron doesn’t evade this drug’s effect. There’s only one 1 mutation for this variant in its main protease and, as would be anticipated, testing paxlovid in the lab demonstrated no diminution of its activity against Omicron.
The 2 clinical trials were presented via press release this week (yes, suboptimal but so far this company’s press releases have matched up with subsequent preprints and publications during the pandemic) .
The results from the randomized, placebo-controlled completed trial of high risk participants is summarized below.
These are striking findings for both the high level of efficacy and safety, and fully consistent with the interim analysis that was first reported with about half as many participants enrolled (unlike the molnupiravar trial which we’’ll get to soon). It is surprising that the benefit was similar at 5 days from symptom onset as compared to 3, since it would be expected there would be attrition over time for the pill intervention effectiveness. Also unexpected was high(er) efficacy in the aged, but the relative risk would also increase and we aren’t yet privy to these details in the results.
The second clinical trial was in standard (lower) risk patients, vaccinated or unvaccinated, and has not yet been completed. It clearly was a lower risk group with 2.4% events (hospitalizations) in the control group compared with 6.3% in the placebo group of the high-risk trial. The relative and absolute reduction in hospitalizations is lower, but quite consistent with the high-risk trial. By definition, this trial is woefully underpowered. With such a low event rate in the control arm, it would actually require a trial many time larger in sample size than the other one to have adequate statistical power.
Noteworthy in both trials was a formal sub-study assessment of viral load quantified as more than a 10-fold reduction (0.93 log10 copies/ml) for Paxlovid, which is impressive and in keeping with the mechanism of action. That also should be pivotal in reducing transmission among people infected, something that seems to have been overlooked at this point.
The Pill Pack and the The Other Pill
It’s going to be a blister pack for 5-days. Twice a day: Two 150 mg tablets of Paxlovid, and One 100 mg of Ritonavir.
That’s right. Paxlovid isn’t given alone. It is accompanied by low dose Ritonavir, another viral protease inhibitor used for HIV, but being used here to increase the blood levels of Paxlovid without any antiviral effect itself. This works through a cytochrome (3A4) that is in common with the metabolism of many frequently used drugs such as statins and certain blood thinners, so a temporary 5-day off such drugs, or adjustment of their doses may be appropriate or necessary.
Moving Forward
The FDA review of the Paxlovid data is imminent and expected to be complete before year end. The emergency use authorization (EUA) can’t happen soon enough. Given the incomplete status of the standard risk trial, that approval is expected to be only for high-risk criteria. Those criteria lack sharp definition, summarized at clinical trials.gov with the main one: “at least 1 characteristic or underlying medical condition associated with an increased risk of developing severe illness from COVID-19.” The high-risk trial ruled out prior vaccination as an enrollment criterion, a feature that may require forethought in the regulatory review since many breakthrough infections will be occurring with Omicron in high risk people.
That brings us to the supply issue since it is expected that only 200,000 treatment course will be available right away, even though the United States has purchased 10 million and Pfizer expects to make 80 million during 2022. That clearly will not be enough given the toll of double dose coronavirus with Delta and Omicron right now, and for what is likely in store next year. All efforts to further rev up production are vital. To achieve this and circumvent dependency on one company, I believe enacting the Defense Production Act would be worth serious consideration. The cost to the government for the treatment courses was more than $500 each but, like vaccines, there will not be a cost passed on to individuals for whom it is prescribed. Obviously, there must be a substantial (as in huge) margin of profit since the cost of goods is minimal.
A New Layer of Defense
Adapting the Swiss cheese model from Professor Ian McKay, illustrated by Rose Wong, a new layer of protection is actualized by this pill on top of all the other layers that we have to work with. Not depicted in this model are rapid antigen tests, which goes along with their relative absence in the United States. Yet they will greatly facilitate the early use of paxlovid and it is essential their availability is scaled and cost greatly reduced (free would be the right cost to promote their frequent use).
What About Vaccines?
The availability of Paxlovid should not in any way diminish the vital need to get all individuals vaccinated and boosted. But people refusing to get vaccinated may consider a 5-day pill treatment as a further excuse to remain unvaccinated. That is yet another reason for the EUA to not stipulate vaccination as exclusionary. In fact, enhanced availability of Paxlovid to people who are vaccinated could be used as yet another incentive to promote more acceptance of vaccines. This is an important issue that needs to be addressed. Moreover, Paxlovid is not a primary prevention, unlike vaccination, it is an intervention for infections, a paramount message that needs to be duly emphasized.
What About Molnupiravir?
The results of the full clinical trial were quite disappointing. What was initially presented as a 50% relative reduction and an absolute 7 per 100 person benefit for fewer hospitalizations at the half-way interim analysis point turned out to be ~30% relative and only 3 per 100 absolute reduction. The second half of the trial essentially had no efficacy, which is peculiar and yet unexplained. The FDA Advisory Committee voted narrowly 13-10 to recommend this drug for EUA, but after more than 2 weeks that has not gone forward by FDA. There are also major concerns about its obligate mechanism of lethal mutagenesis for the virus, and what the implications that has for unleashing dangerous mutations. The idea of combining this pill with the Paxlovid /Ritonavir has been brought up, but there are no data for that and it would just make things more complicated.
Summing Up
Paxlovid is a breakthrough, and I don’t use that term lightly. It not only provides a new layer of defense, but a unique one that is not dependent on our immune system and not affected by Omicron or other variants. It is the first drug specifically designed for SARS-CoV-2, not a repurposed molecule, and its efficacy supports that specific effort. Of course it would have been terrific to have it available from the earliest days of the pandemic, but the speed with which this molecule has been tested and validated is indeed impressive. We need it out there in large quantities ASAP. I cannot adequately emphasize its potential salutary impact.
P.S. One last note for context
To remind you, as I stipulated in my Welcome post, I have no relationship with Pfizer. In fact, along the way of the pandemic, I have taken on this company multiple times for their lack of transparency for not promptly releasing their vaccine trial protocol (finally released after intense social media pressure) for their CEO broadcasting their vaccine trial interim analysis plans to apply for an EUA after the first 32 events (trial protocol called for 150 events), for their announcement in April 2021 that we would all need booster shots when there were no data to back that up, and many other issues. That is, I have a bias against this company.