Why the new RSV vaccines are a BFD
The era of rational, structure-based design of vaccines that's hitting it out of the park.
It has taken over 65 years to get to this point, but, remarkably, new major triumphs, built on decades of work, have attracted little attention to date.
This is about Respiratory Syncytial Virus (RSV):
—Each year about 120,000 children die from RSV, half are under age 6 months.
—For children younger than age 5, there are 33 million cases of bronchiolitis/ pneumonia and 3.6 million hospital admissions worldwide. A large proportion are in low and middle income countries.
—For older adults in the United States, there are about 100,000 hospitalizations and 8,000 deaths associated with RSV. It rivals seasonal influenza for morbidity and mortality in older adults.
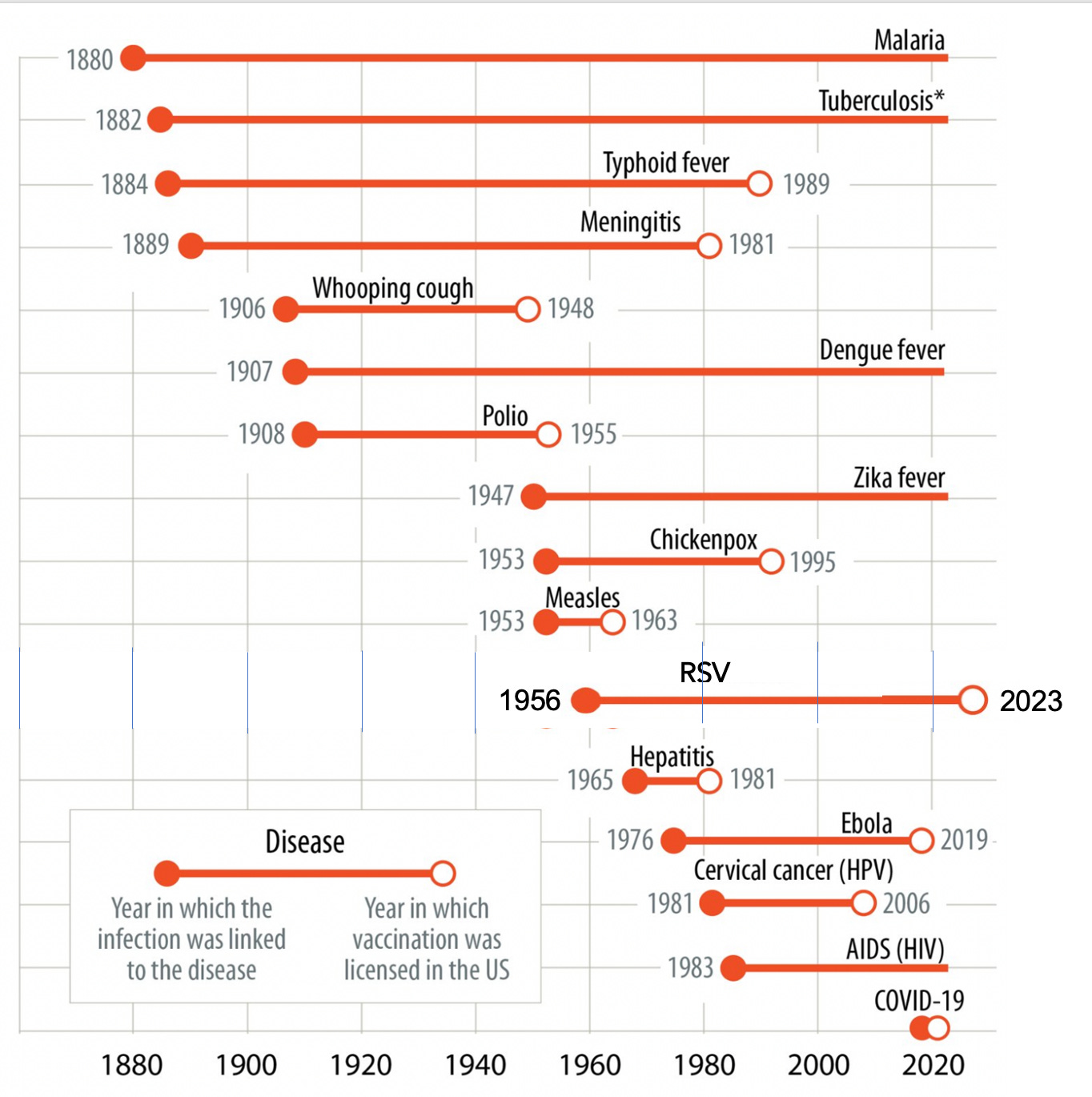
—The pathogen was first identified in 1956 in Baltimore but we have not had a successful vaccine until now.
In the past several weeks, 4 randomized trials of RSV vaccines in pregnant women and older adults have been published in the New England Journal of Medicine. Recall it takes an average of 8-10 years from the identification of the causative pathogen to a successful vaccine (emphasis on the successful category). And for many pathogens, as shown in the graphic below, adopted originally from Max Roser and Edouard Mathieu at Our World in Data, there are no vaccines—like malaria, TB, Dengue fever, and HIV, up to 140+ years later! I have added the recent success story for RSV to put it in context—the 67-year journey. Of course, none of these long incubation periods compare with the Covid vaccines which were developed and validated in just 10 months, thanks to relentless science advances of mRNA and nanoparticles, and structural biology, for over three decades. As it turns out, we’re seeing the windfall of that seminal work pay off for RSV and several other pathogens.
The Data from 4 Randomized, Placebo-Controlled Trials of RSV Vaccines
The four trials were all 1-dose vaccines from 3 different manufacturers: Glaxo Smith Kline (GSK), J&J, and Pfizer. The GSK trial was in ~25,000 participants age 60+ (mean age 70). One dose led to a 94% efficacy against severe RSV pneumonia and 72% efficacy against RSV acute respiratory infection (graph below). There are 2 RSV subtypes, A and B, with higher efficacy against A (81-85%) as compared with B (70-72%)
The J&J (Janssen) trial enrolled nearly 5,800 people age 65+ and demonstrated one dose efficacy between 70-80% depending on definition, with 80% for the most severe RSV disease.
The Pfizer adult trial, the largest RSV vaccine trial with over 34,000 participants, was also conducted in adults age 60+, has a similar high rate of efficacy ranging from 67% to 86% depending on the severity of the RSV pneumonia, with again higher protection against the most severe form.
The 4th trial was also with the Pfizer RSV vaccine in nearly 7,000 pregnant women and showed an efficacy of 82% at 90 days after birth that was maintained at 69% 6 months after birth for severe RSV on their infants, as shown below.
To note, in all 4 of these trials there were no safety concerns identified besides the expected acute phase reactogenic side effects as seen with the Covid mRNA/nanoparticle vaccines.
The Secret Sauce
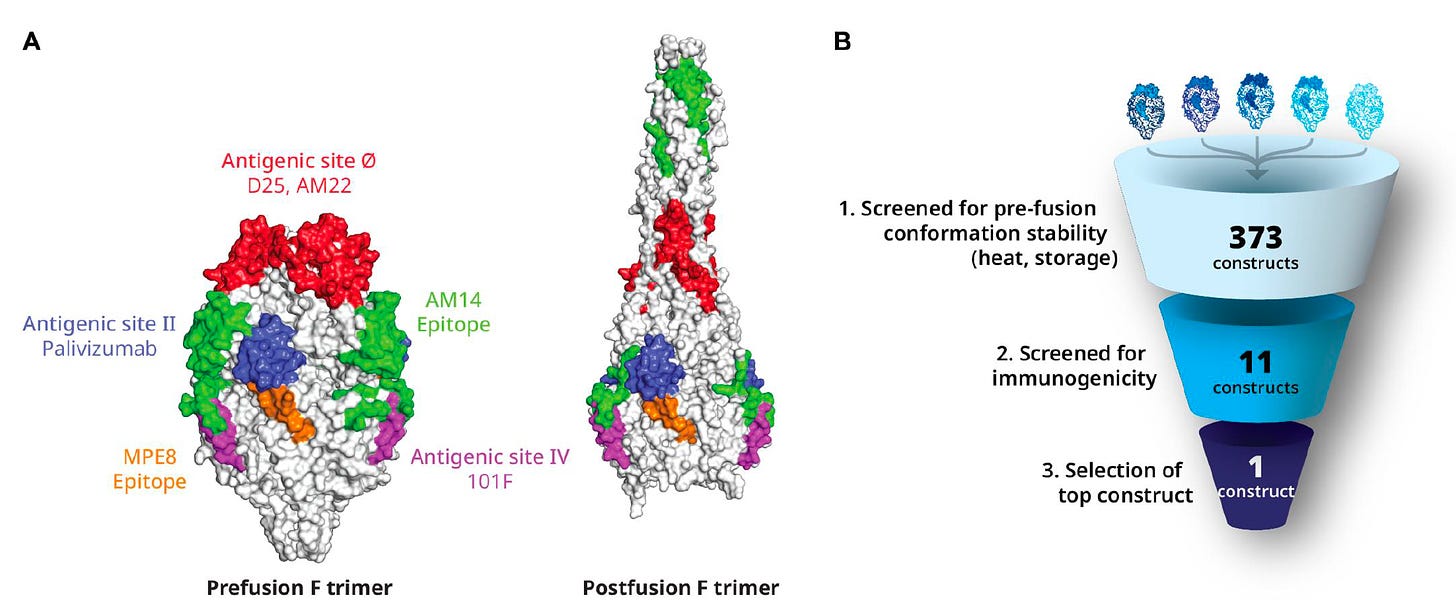
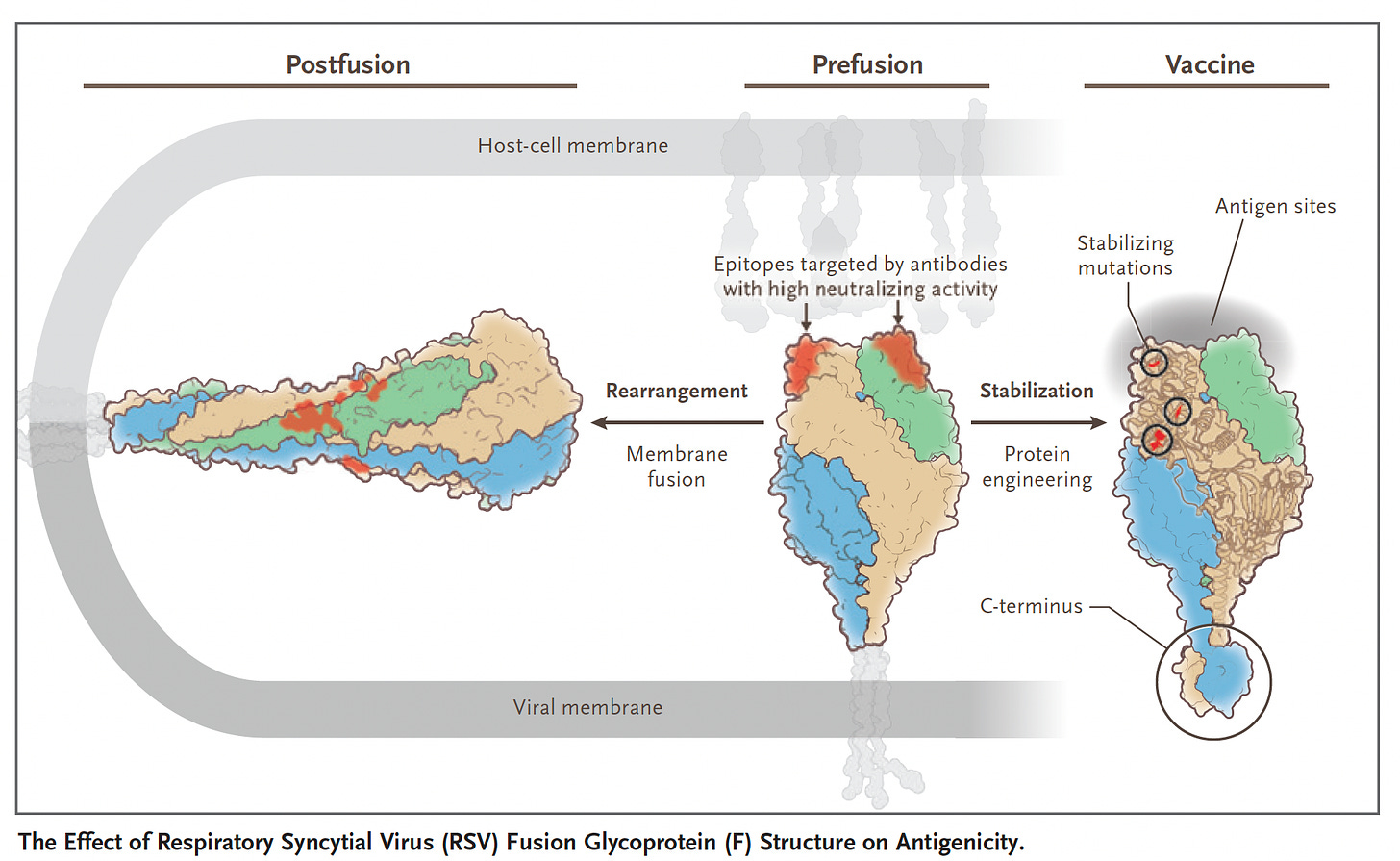
It’s about structure-based design of the RSV vaccine, which got a giant leap in 2013 with the publication of a paper in Science by Jason McClellan and colleagues at the NIH Vaccine Research Center with determination of the atomic-level structure of the RSV fusion (F) protein (Think of F like the SARS-CoV-2 spike S protein, that drives the membrane fusion of the virus and the host cell). This work unraveled the importance of the prefusion F, eliciting higher neutralizing antibodies than wild-type RSV F, using the DS-Cav1 antigen (shown below). But the prefusion F, which became the principal target for a RSV vaccine, is chemically unstable, so it took considerable work to rise above this challenge: to make a stable, highly immunogenic prefusion F antigen. I should add this crystallization of proteins back in 2013 was painstaking, laborious, multi-year work that couldn’t be rapidly accomplished with the help of AlphaFold2, the AI platform developed by DeepMind that now rapidly (minutes to hours) and accurately predicts 3D structure from amino acid sequences. It is noteworthy that AlphaFold2 was used to come up with a whole new delivery method of proteins into cells, a “molecular syringe” system, as published by Feng Zhang and colleagues in Nature last week
The extensive back story of the Pfizer protein-based vaccine’s development to meet this challenge was described in a Science Translational Medicine paper this week with the graphic below.
Barney Graham, a key player at NIH in the RSV vaccine (and many others) development, wrote an editorial accompanying the first 2 RSV vaccine trials in NEJM, with the graphic below, pointing out the hyper-accelerated success of Covid vaccines were an outgrowth of the RSV vaccine structure-based design, which relied on stabilizing the pre-fusion protein (S, spike) with a 2-proline substitution to maximize the immunogenic response: “work that had taken decades in RSV research was compressed into just a few weeks for SARS-CoV-2.”
This, simplified, is beating out nature, rationally designing a better antigen (with amplified immune response) than the wild-type, be it the preF, used in all the RSV vaccines, or the 2-proline S substitution incorporated in nearly all the Covid vaccines. It’s the essence of atomic structure based design of vaccines and monoclonal antibodies.
Why this is a BFD
This —mRNA/nanoparticle delivery of structure-based vaccines— has set up multiple success stories across so many pathogens that were previously not approachable, as nicely reviewed in this article and graphed below. This now even includes, for the first time, a universal flu vaccine that was effective against all 20 A and B subtypes in multiple experimental models.
Beyond that, rational antigen structure-based work towards a Hepatitis C vaccine is gaining traction. We’ve gone from HCV’s discovery as pathogen in 1989 without a vaccine, and its success would have a huge potential impact for decreasing chronic liver diseases and liver cancer—against about 300,000 deaths per year worldwide—as summarized in this week’s Science.
In a previous post, I’ve reviewed how this science is now accelerating individualized vaccines for cancer, against autoimmune disease such as multiple sclerosis, and more.
This is the most exciting time in the history of vaccines. It took many, momentous, sequential discoveries over at least 3-4 decades to get here. That we were able to get Covid vaccines in 10 months from sequencing the virus with over 70,000 participants in randomized trials and 95% efficacy vs symptomatic infections (and hospitalizations and deaths) is all too often taken for granted. I had never thought that would have been possible, but now I understand how it was achieved. It has been exhilarating to see all the work from many labs around the world culminate in such a rapid succession of success stories, with many more to come.
Thanks for reading Ground Truths. Please share. Subscriptions are free and, of course, welcome. They tell me these newsletters are helpful to you.
You will not be getting notices of Ground Truths via Twitter as Substack links have been blocked. Another reason to subscribe!
Reminder: the proceeds from all paid subscriptions are donated to Scripps Research.
N.B. I have no relationship with J&J, GSK, Pfizer, Moderna or any vaccine manufacturer mentioned in this piece (and beyond that in any previous writing).