A one-two punch against pancreatic cancer
A.I for predicting high-risk and a promising vaccine in a clinical trial
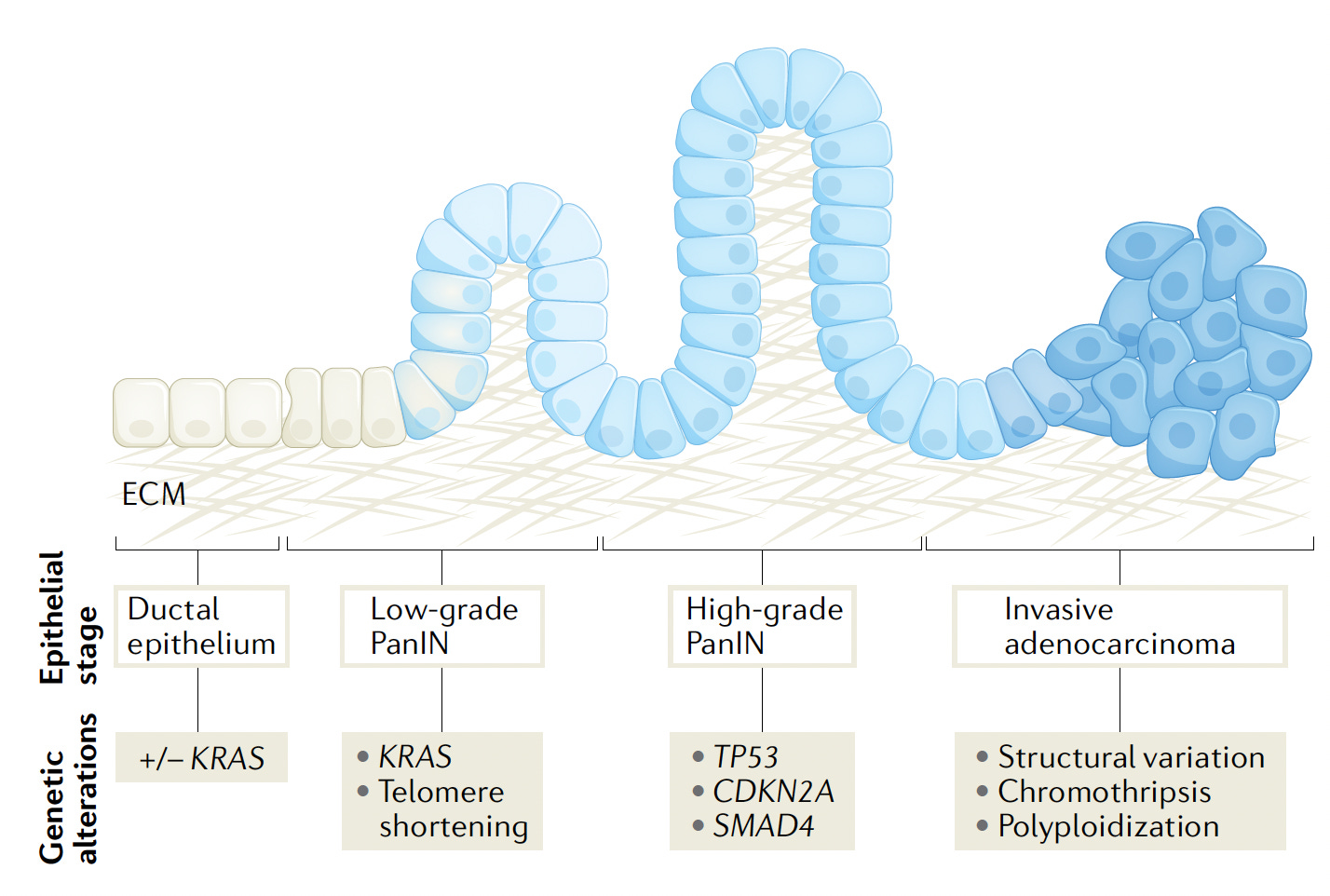
Pancreatic cancer is one of the deadliest, with a 5-year survival of less than 10%—that hasn’t changed in several decades. It is the cause of approximately 50,000 deaths in the United States each year which ranks third after lung and colon cancer. Patients with pancreatic cancer typically present late in their course, when there are high-grade intraepithelial neoplasms (PanIN) or invasive adenocarcinoma, as schematically shown below, along with the characteristic genetic alterations. A review in The Lancet emphasized “New strategies for screening high-risk patients to detect pancreatic tumours at earlier stages are desperately needed to make a clinically significant impact.”
A.I. to Predict High-Risk
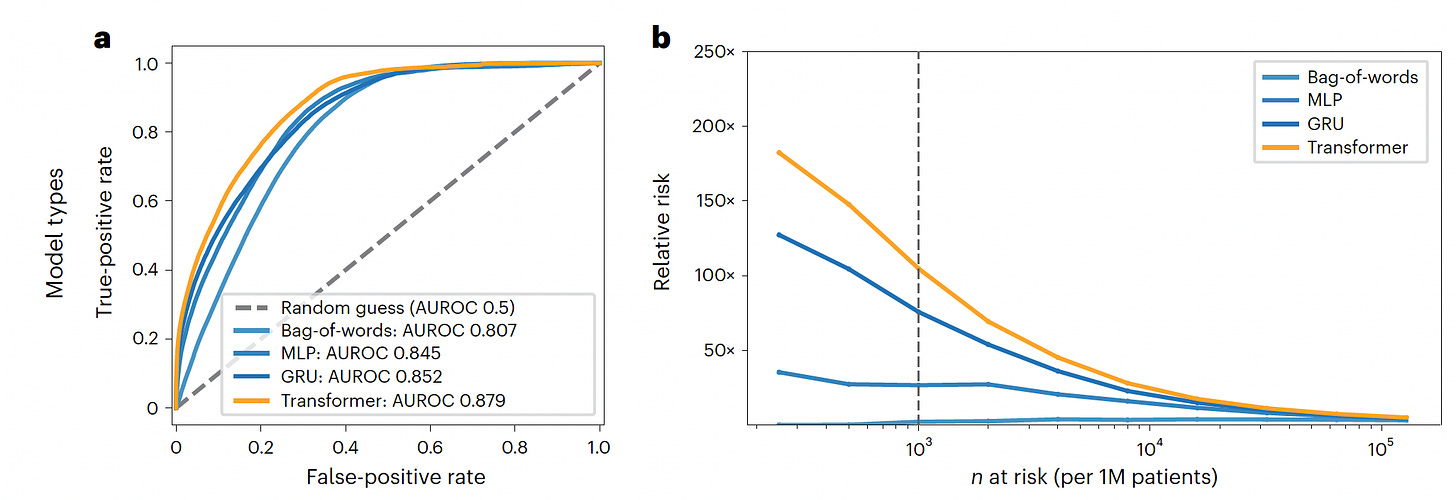
This week a major study was published using deep learning A.I. for predicting high-risk individuals for developing pancreatic cancer up to 3 years in advance of diagnosis. This work involved 2 distinct cohorts, one from Denmark and the other from the Veterans Affairs in the United States, in aggregate nearly 28,000 patients with pancreatic cancer. The data inputs from longitudinal medical records over several years were used, which provided the added dimension of time sequence. The algorithm development for risk prediction included use of a Transformer model, the basis for generative A.I., well suited for the time sequence data, and had the best performance (AUC=0.88).
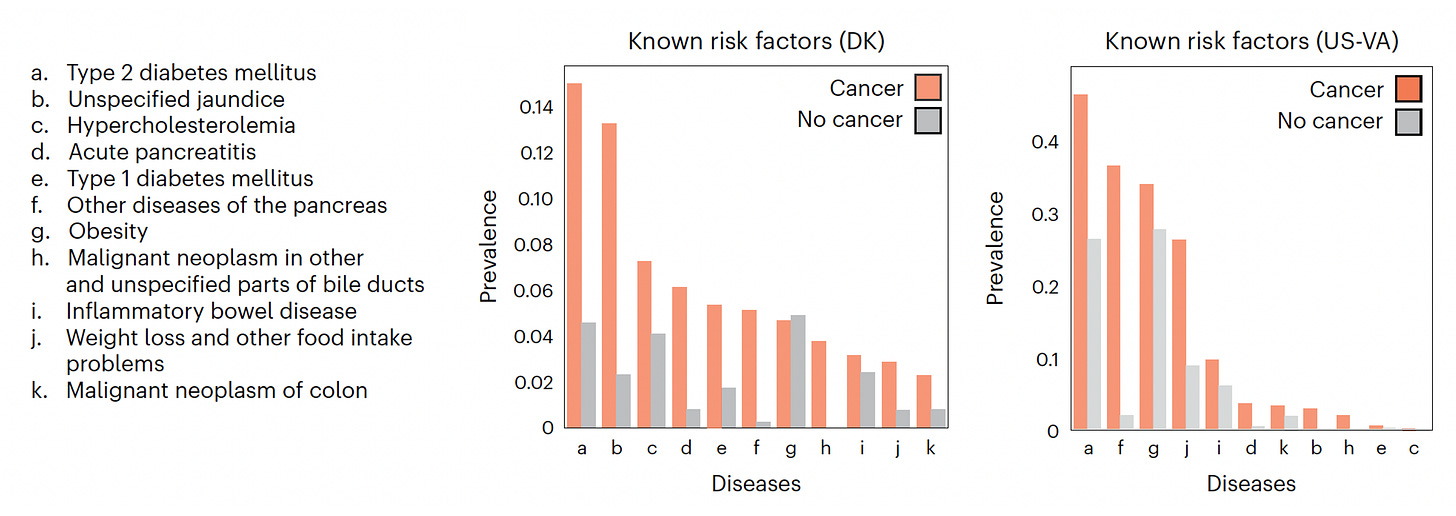
Below you can see the proportion of known risk factors present in the 2 cohorts (Denmark-DK, US-Veterans Affairs) for those who developed pancreatic cancer and the near 11 million patients who did not.
The graphs below show the Transformer model performance compared with the others tested (bag-of-words, MLP, GRU), both with respect to the area under the receiver-operating characteristic, AUROC) and picking up the relative risk per million patients. This model was better at short-term prediction in 12 months compared with 3 years, and performed better in the Danish dataset than the Veterans cohort. Most of the top 10 features that contributed to model prediction were similar in the 2 datasets, but some were only found in one, such as cataracts or dermatitis in the VA cohort, or anemias in the Denmark cohort. Of note, this model with “just” longitudinal medical records identified the highest-risk group, such that of the 1,000 patients age 50+ , 320 would develop pancreatic cancer. Of course, these important findings will require independent replication and extension. It is quite likely that the model performance can be improved with multimodal AI, incorporating layers of data such as a person’s genome and gut microbiome, laboratory data (even trends in the “normal range”), and many other features not assessed in the current study including cell-free tumor DNA from a blood sample (also known as a liquid biopsy).
A Neoantigen Vaccine Works
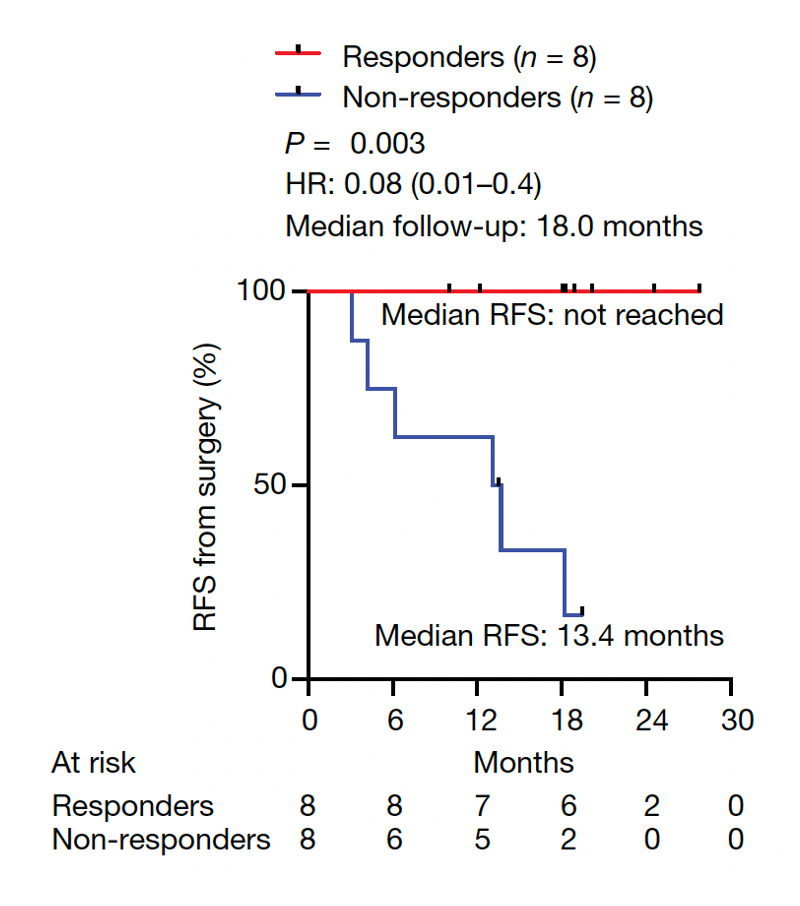
In the same week in the journal Nature results from a small clinical trial of an individualized mRNA neoantigen vaccine were published. Previous efforts for immune-checkpoint inhibitors (ICI) for pancreatic cancer have not generally panned out. An alternative way of revving up a person’s immune system is to identify the neoantigens (new proteins on the cell surface) from tumor tissue by sequencing DNA and RNA. Up to 20 neoantigens were identified for each patient and incorporated in a mRNA-nanoparticle vaccine given 9 weeks after surgery (all patients received an anti-PD-L1 immunotherapy, an ICI) . Half of the 16 patients were responders with a marked T-cell response (predominantly CD8+ cells, capable of direct killing of tumor cells) to the neoantigens and they had no evidence of cancer at a median of 18 months follow-up. The simplified schematic below summarizes the study.
It’s a small Phase 1 trial but one that is very challenging for designing individualized vaccine and assessing the immunologic and clinical outcomes. It is without a placebo-control group, making all conclusions tentative. It builds on an important case report of a 71-year-old woman with refractory, widely metastatic pancreatic cancer who initially showed a marked response to an infusion of engineered T cells. It was noted in the present study that one patient appeared to have disappearance of a liver micrometastasis resulting from the T cells induced by the vaccine. We don’t yet know why half the patients did not have a T cell response to the vaccine. Nevertheless, the relapse-free survival (RFS) was significantly different for the responders with marked T-cell response specific to the neoantigens. It’s a preliminary finding but certainly supports this may be a promising approach for treating patients with advanced pancreatic cancer. A large scale trial testing this strategy is starting soon.
The NY Times coverage of this report gives more background on how it was accomplished, involving collaboration with BioNTech and Genentech, and pointing out that cost is a major barrier. Dr. Vinod Balachandran, the senior author of the Nature paper, has posted a very good (and long) thread on this work
Bookends
These 2 studies can be seen as “bookends” for new approaches to pancreatic cancer, one to identify high-risk patients for surveillance, the other to potentially treat patients with refractory, life-threatening cancer. Both can be seen as precursors for what is to come. Better A.I. models for defining high-risk that could be used in conjunction with serial liquid biopsy surveillance (as I explained previously) could detect pancreatic cancer at the earliest, microscopic stage. For patients who break through early detection, we may finally be on to a way to exploit an individual’s immune response to effectively take on this aggressive cancer.
Since we have seen so little progress for this most vexing form of cancer, it is encouraging to see this new work on both ends of the spectrum from enhancing early diagnosis to treatment. I’d like to think it represents the foundation for a significant step forward. Perhaps you have had a family member or friend succumb from pancreatic cancer and will well recognize how, with so little to offer, there is so much room for improvement. We’ve got some hope here.
Thanks for reading, subscribing and sharing Ground Truths.
As I recently conveyed, I’m indebted to the paid subscribers who helped fund many summer interns—high school and college students—at our Scripps research program. All proceeds from Ground Truths go to Scripps Research.