The fascinating and evolving story of bacteria and cancer
From in the tumor to tackling the tumor
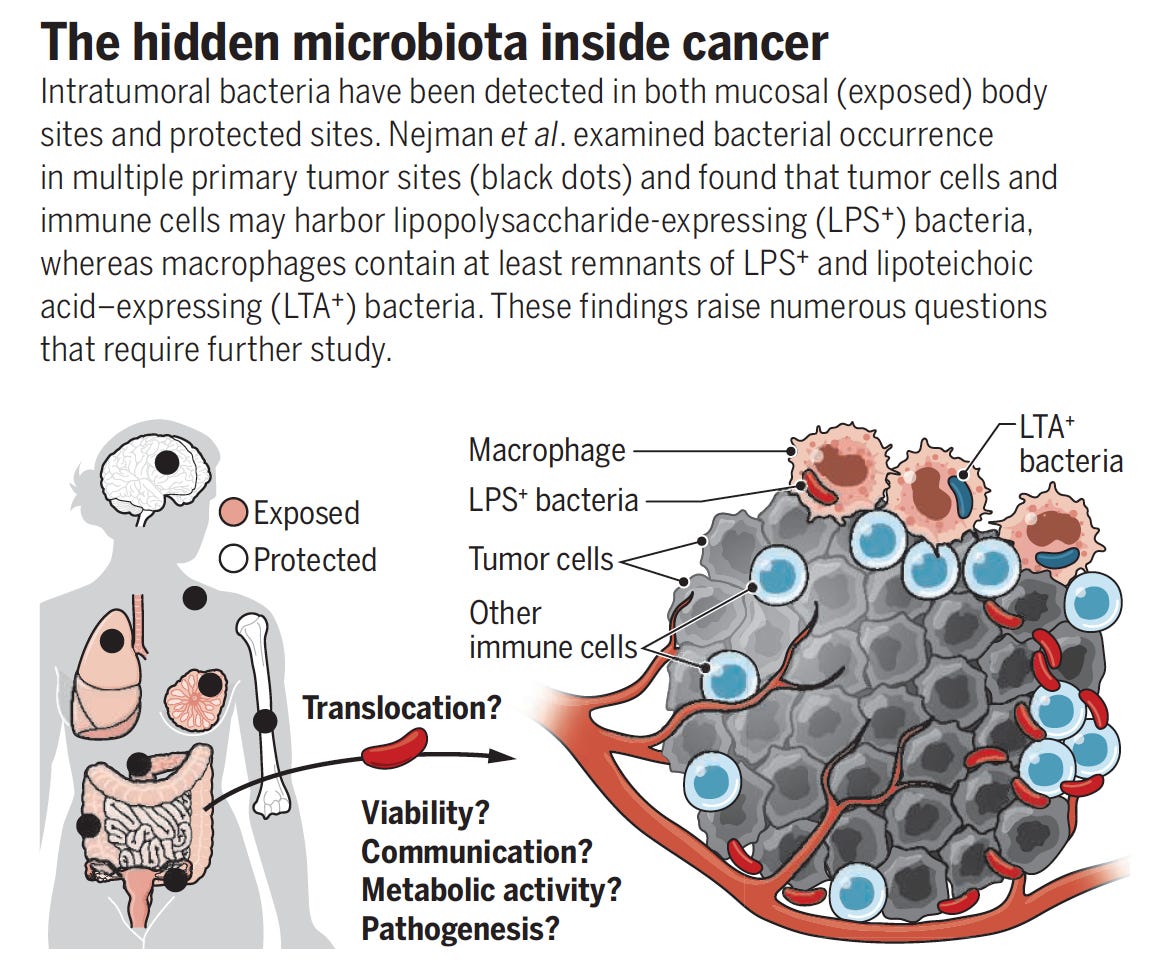
It was medical dogma: cancer tissue is sterile. That’s what we had learned and taught in medical school for decades even though bacteria were detected in tumors more than 100 years ago. When studies were reported asserting that bacteria were present in tumor tissue, they were consistently debunked as representing contaminants. Then came new tools that include single-cell sequencing and sophisticated spatial profiling (I’ll describe more on this later) providing high-resolution portraits of tumors. The new dogma is that bacteria have a pervasive (yet variable) presence within and across solid tumors—the ”presence of intratumoral bacteria being designated a hallmark of cancer.” Furthermore, where bacteria are more apt to be found within tumor regions, T cell recruitment and function is suppressed. These regions of tumor are micro-niches exhibiting immune evasion.
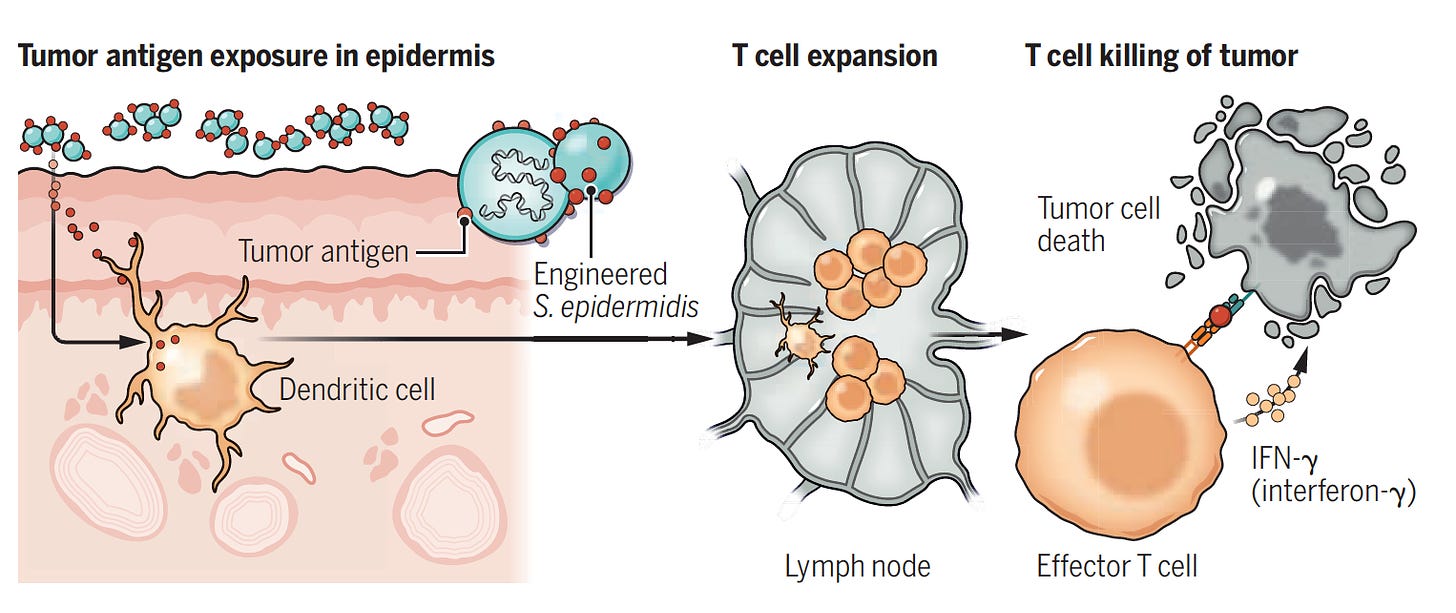
Just as that has been determined, there was a new twist this week: engineering bacteria to induce a potent T cell immune response to kill the tumor. This can be viewed as the polar opposite. Instead of bacteria improving a tumor’s ability to duck our immune response and spread, this represents clever ways to genetically manipulate bacteria (aka “designer bugs” with the schematic below) to make it considerably more antigenic, a new route to immunotherapy.
Now let me get into the details of these 2 major new fronts of knowledge for the intersection of bacteria and cancer.
Bacteria in Tumors
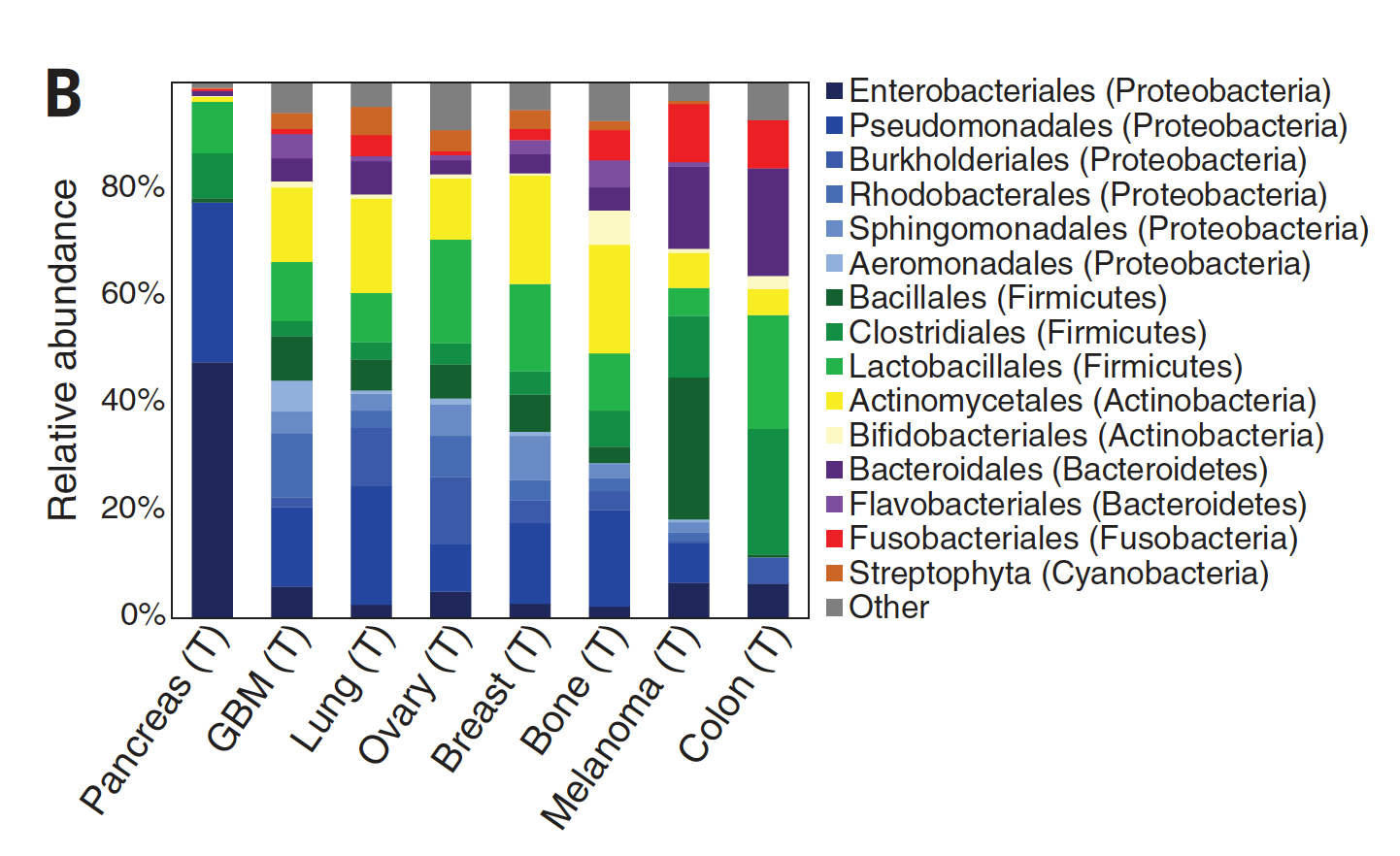
While there was considerable debate about the extent and localization of bacterial presence in cancer, a seminal study published in Science in 2020 changed that. A comprehensive assessment across 7 cancer types, from over 1500 tumor samples, showed the presence of bacteria, mainly inside cancer and immune cells. Of the solid tumors assessed, breast, bone and pancreas cancer had the highest estimates of bacterial load.
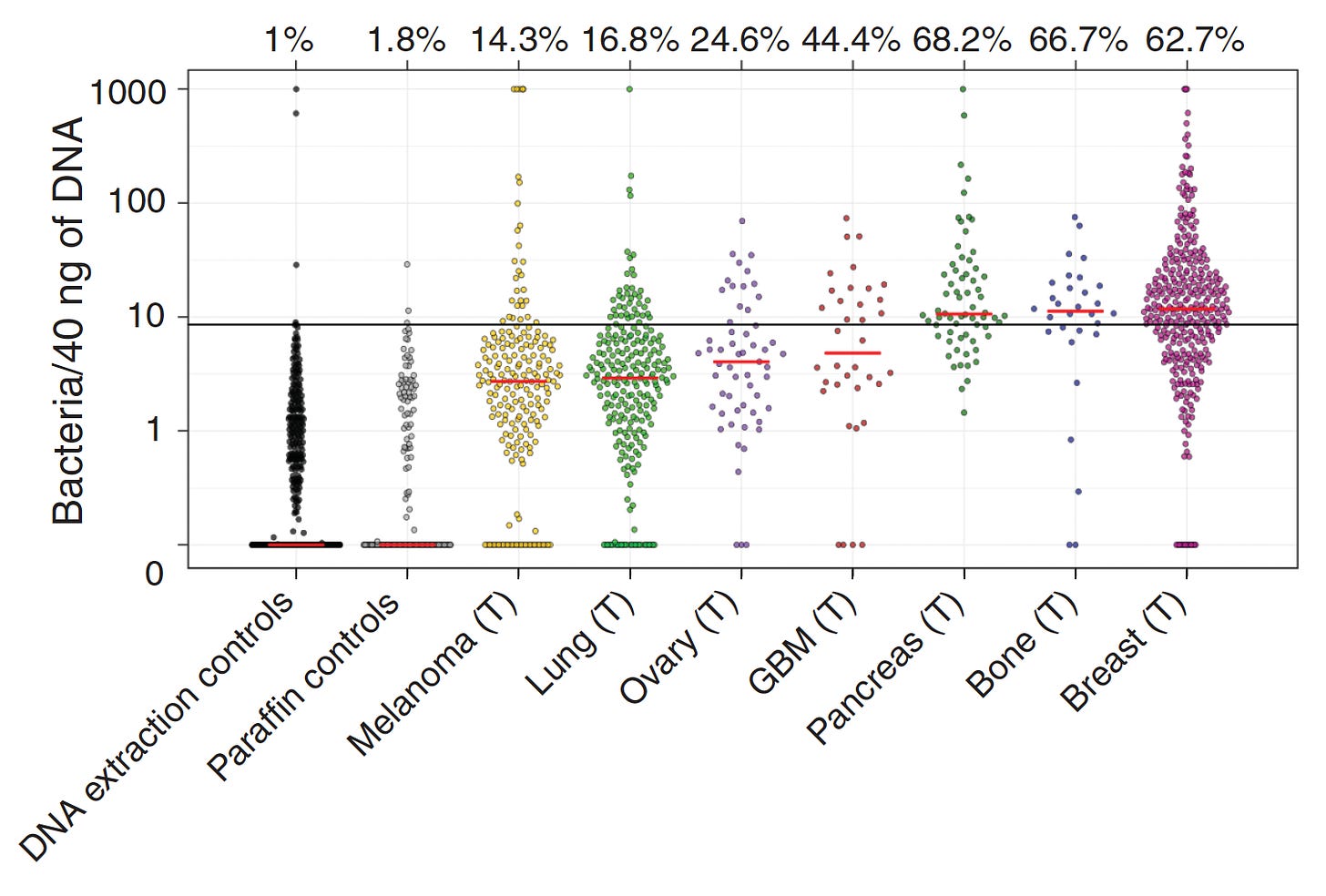
Specific bacterial species were correlated with cancer types as shown below. Other reports since this publication have demonstrated that virus and fungi can be present within tumors, but their frequency and significance is less known.
A good summary of these important findings here with the graphic below.
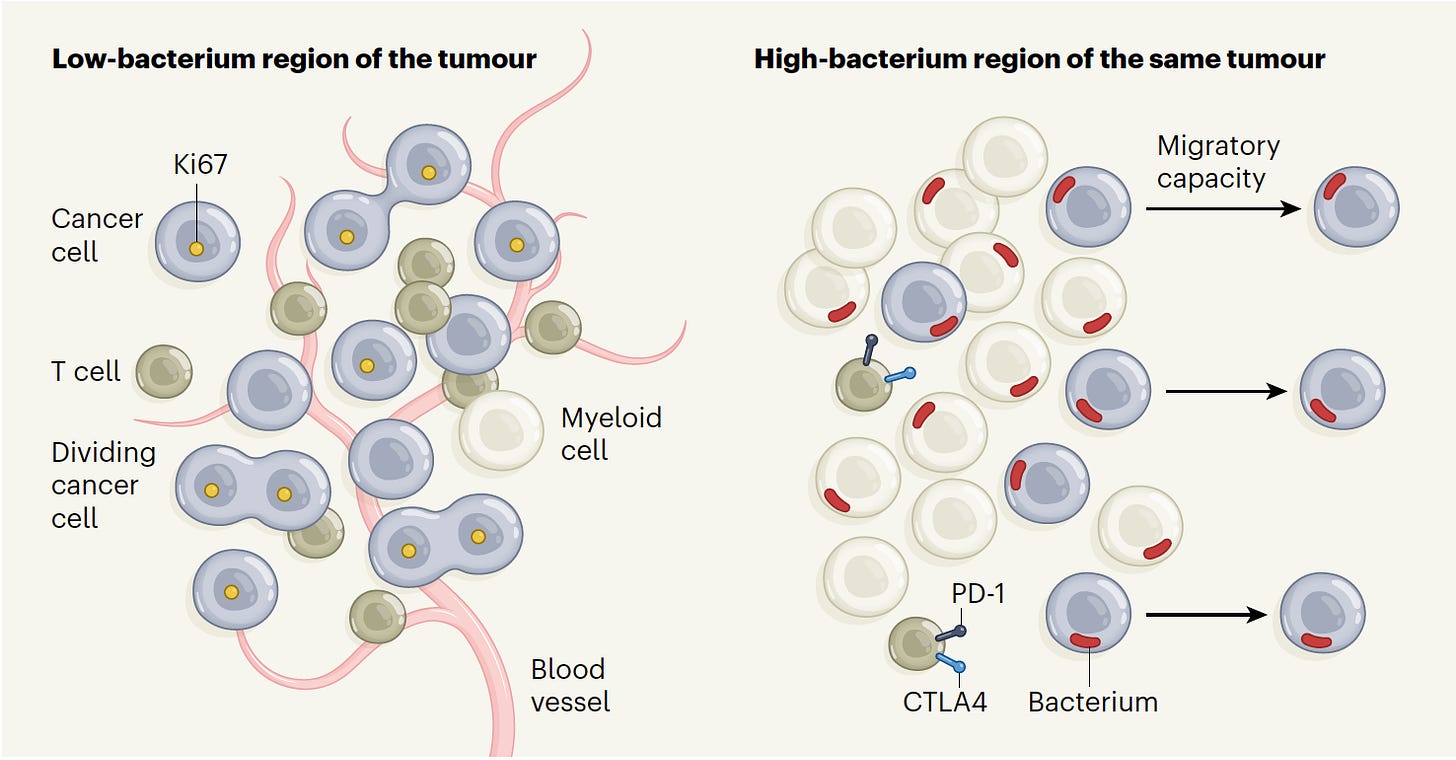
That study firmly established the presence and localization of bacteria in diverse solid tumors, and a more recent important study took this a big step further providing the spatial profiling of bacteria in colorectal and oral squamous cell tumor regions, within cancer and immune cells. This was accomplished with high-throughput in situ spatial transcriptomics, which quantified bacterial loads, demonstrating the substantial heterogeneity in cancer tissue, with niches that were categorized high-bacterium as schematically shown below. Multiple state-of-the-art spatial profiling methods were used, including RNAscope (visualization of bacterial RNA within individual cells), GeoMX (digital spatial profile with 77 antibodies to define protein characteristics), 10X Visium (RNA transcripts with known spatial location), and INVADEseq (human and bacterial RNA from the same cell). The cells infected with bacteria had increased expression of pathways known to enhance cancer cell invasion and metastasis. In the bacterium-colonized regions, the depleted T cells were indicative of niches of immunosuppression. Professor Susan Bullman, the senior author of this work, has an excellent summary in the new issue of Cell this week.
Bacteria Killing Tumors
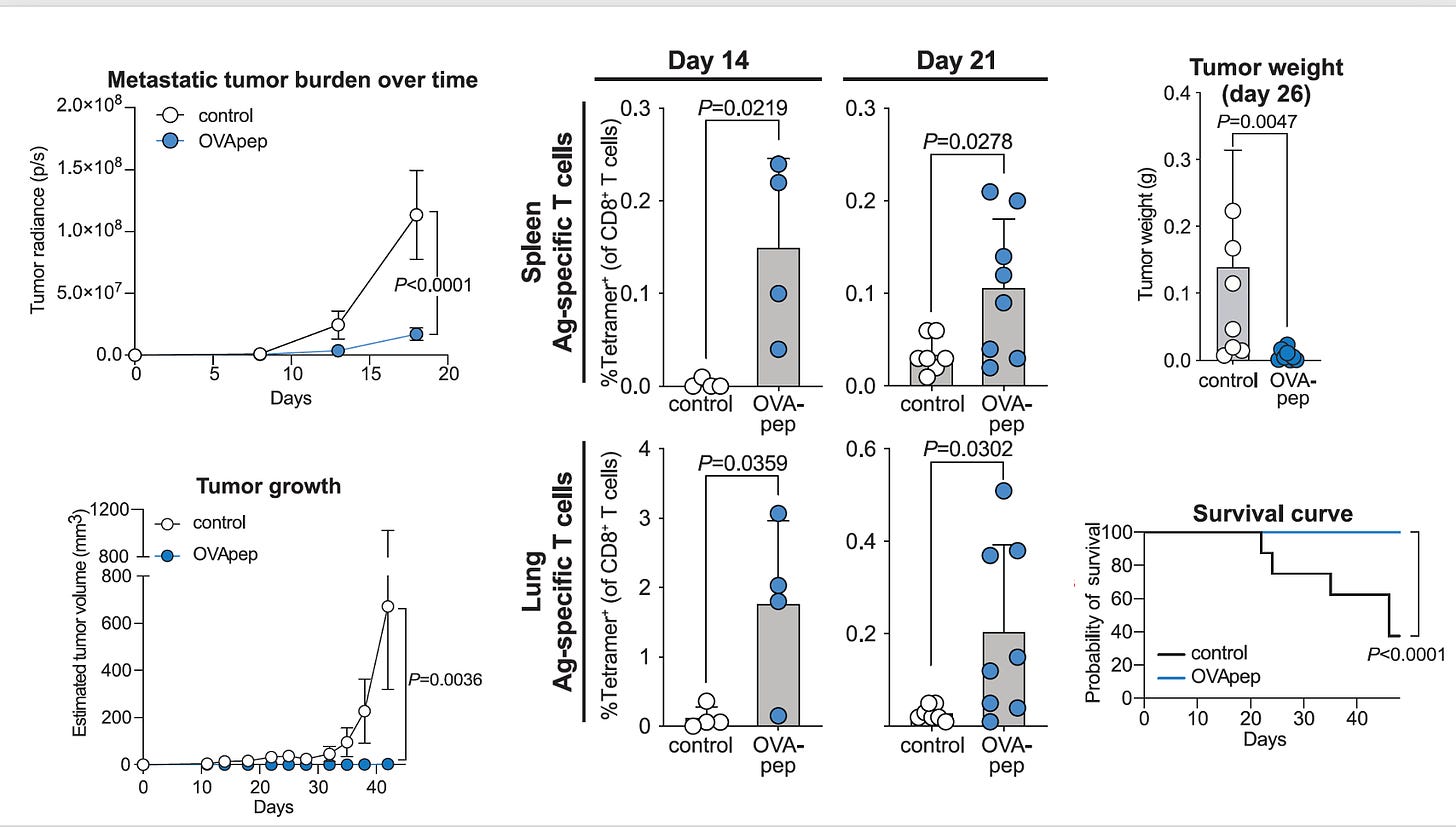
Akin to my recent post of discovering a better-than-natural antigen with RSV or SARS-CoV-2 for vaccines, this is about manipulating the genome of a skin bacterium (Staphylococcus epidermidis) to make antigens that elicit a potent T cell response against melanoma. These enhanced T cells were capable of migrating to distant skin melanoma graft sites well beneath and beyond the skin, including the metastases in the lung, with a very strong anti-tumor killing property—without causing inflammation. Notably, this approach did not result in infections, did not require intratumor delivery, and appeared to be synergic (more than additive) with immune checkpoint therapy. A series of experiments in the mouse model, as represented below, reinforced how effective the OVA-pep (ovalbumin-derived S. epidermidis constructs of full peptide), was relative to control for tumor burden, tumor growth, T-cell response, tumor weight, and survival. If the engineered antigen was not present, these salutary effects were not seen. This can be seen as a potential new means of immunotherapy for cancer and more broadly a novel pathway to modulate our immune response.
These two new frontiers, represent almost mirror images—bacteria in tumor cells that enhance immune escape, in contrast to manipulating bacteria to rev up the immune response. Of course, they are not the only way bacteria intersect with cancer. In recent years, we’ve had ample evidence of how the gut microbiome influence cancer treatments, particularly immunotherapy.
That was “old” news back in 2017. The new ways of bacteria and cancer interacting—-in both bad and good ways—is an exciting work in progress, that with enhanced understanding, may well lead to improvements in therapy. We’ve come a long way from thinking cancer tissue was sterile, to a fertile field that can even exploit bacteria, discover antigens that beat nature’s immune response, and perhaps in ways that will transcend cancer in the future.
Thanks for reading and subscribing to Ground Truths. If you find the post of interest, please share widely.
The proceeds from all paid subscriptions will be donated to Scripps Research.