The reluctance for Americans to get a booster shot has been striking. The United States currently ranks 73rd among countries for its uptake of boosters at 33% of its population. All peer, rich countries around the world are at least double that rate. Countries ranking above the US now include Rwanda, Uzbekistan, Iran, Honduras, and Azerbaijan. Seemingly, you’d have to work very hard to show up this poorly as the country that first validated the vaccines, manufactures them, and has had such a surfeit supply that it has >50 million shots it can’t get anyone to take. Nonetheless, it has maintained optimism and purchased 171 million new Omicron BA.5 variant bivalent shots.
There are many reasons for this abject failure—a veritable booster botch—stemming back to the beginning of the US booster campaign plan in August 2021, with mass public confusion induced by a different plan announced every few days and infighting between the different governmental agencies (CDC, FDA, NIH, WH) as to the appropriate strategy. This was compounded by the very late endorsement that boosters are necessary for all adults that did not come until the end of November, even though the data from Israel and other countries were clearcut many months prior to that juncture. Delays, confusion, and poor messaging got boosters off on the wrong footing. All the anti-science, anti-vax, mis- and disinformation hasn’t helped at all, and has never been effectively countered.
Incontrovertible Evidence for Booster Benefit
Very strong evidence supporting boosters dates back to October 2021, when the results of the only large (~10,000 participant) (1st) booster randomized trial were released and later published, with a 95% reduction of symptomatic infections across all age groups, through the Delta wave, durable at that level for at least 4 months. There were no safety concerns or myocarditis. The efficacy level was fully restored to the original randomized trial (95%) reports in November 2020.
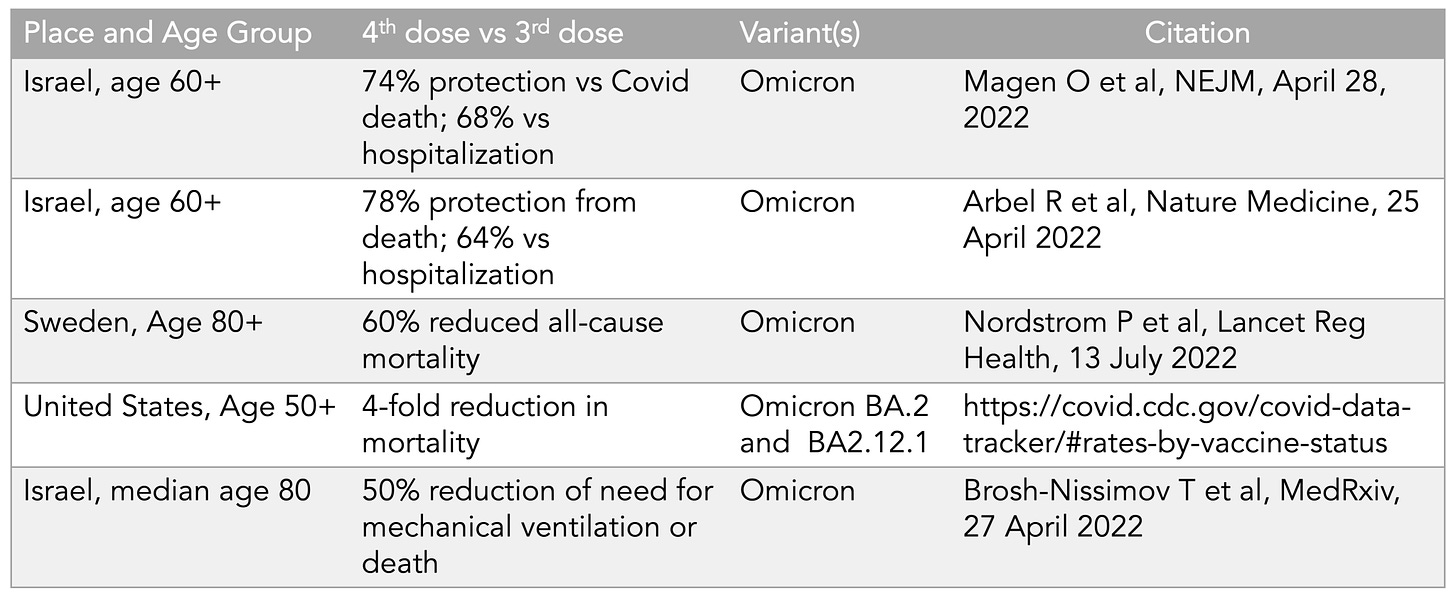
Building upon the evidence for a 3rd dose were many vaccine effectiveness studies, and subsequently there have been 5 reports showing the benefit for the 4th dose vs those who had received a 3rd dose for reducing deaths, summarized in this table, for age 50+.
That’s a big relative reduction of death that has similarly been shown for hospitalizations. There’s also some evidence for reduction of Long Covid with a booster with another new report that reinforced the ~50% protection from Long Covid via vaccination. However, since the Omicron wave (BA.1, BA.2, BA.2.12.1, BA.4/5) there has generally been less protection against infection and transmission from boosters and vaccines, down to levels of 30 to 40% in the first 2 months, and less durable. That’s been a disappointment that has further detracted from enthusiasm for boosters. There’s also the reactogenicity, which I’ve experienced with 2nd, 3rd and 4th shots and which I would not want to keep having for 1-2 days of being knocked out, with profound fatigue, headaches, chills, etc. Who would want to sign up for that without assurance of important enhanced protection?
With this background, it is understandable that even the new BA.5 bivalent boosters, which nicely match up with the current circulating variants (BA.5 88%, BA.4.6 10%) would not be highly alluring, as reflected in recent headlines
The US rollout of the BA.5 bivalent boosters started on Friday September 2nd, and was predicated on 3 sets of data: the BA.1 bivalent vaccine, the Beta variant bivalent vaccine, and mice data. I’ve previously written about my reservations of going forward without human BA.5 bivalent vaccine data (and not using a monovalent BA.5), particularly in light of poor uptake of boosters in this country and the public optics of not having data to nail down the immunologic response. We’ll have those data soon, in the weeks ahead, but had the companies been given an ultimatum in June to have such data by September we’d already have had that in hand.
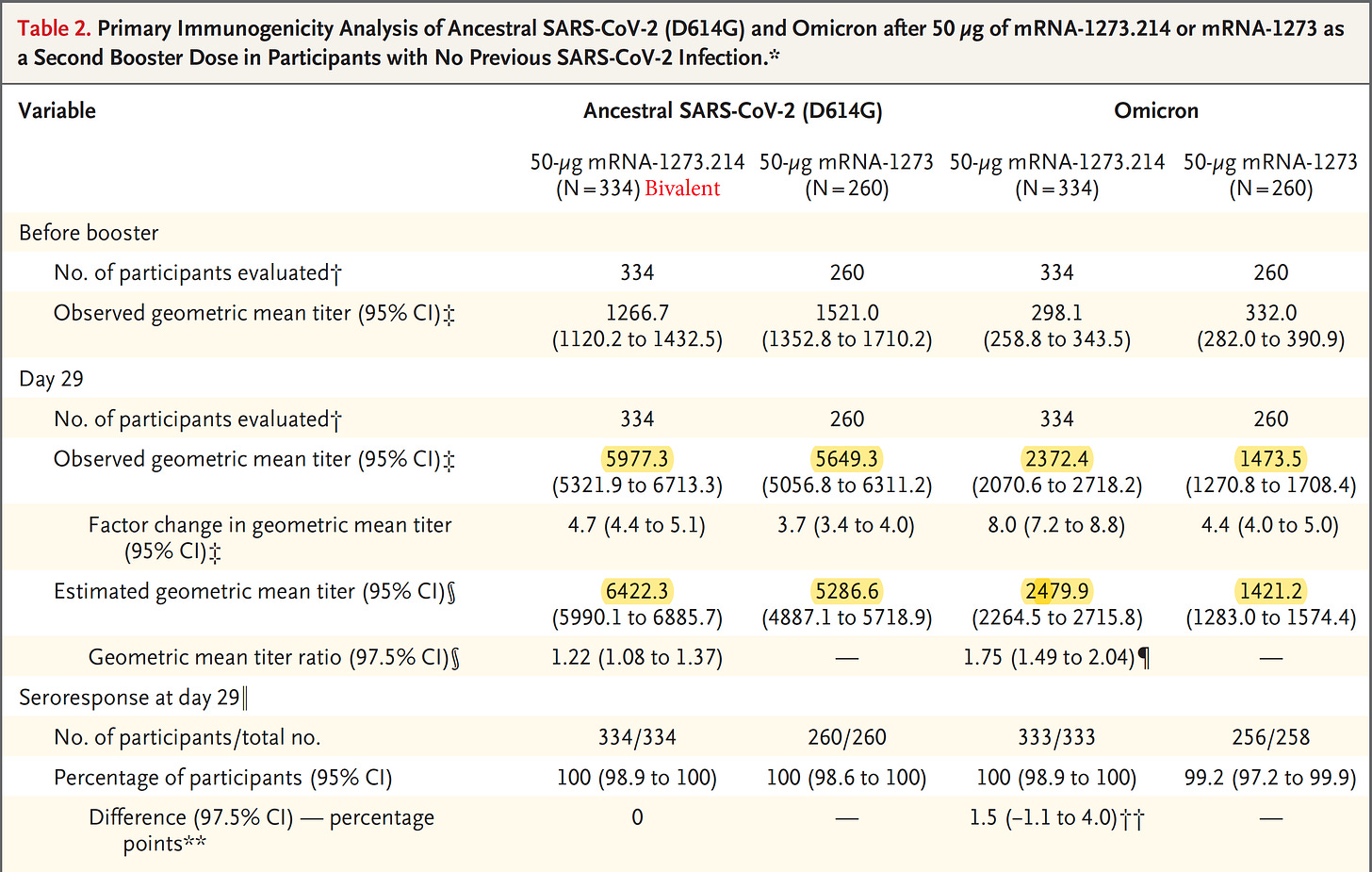
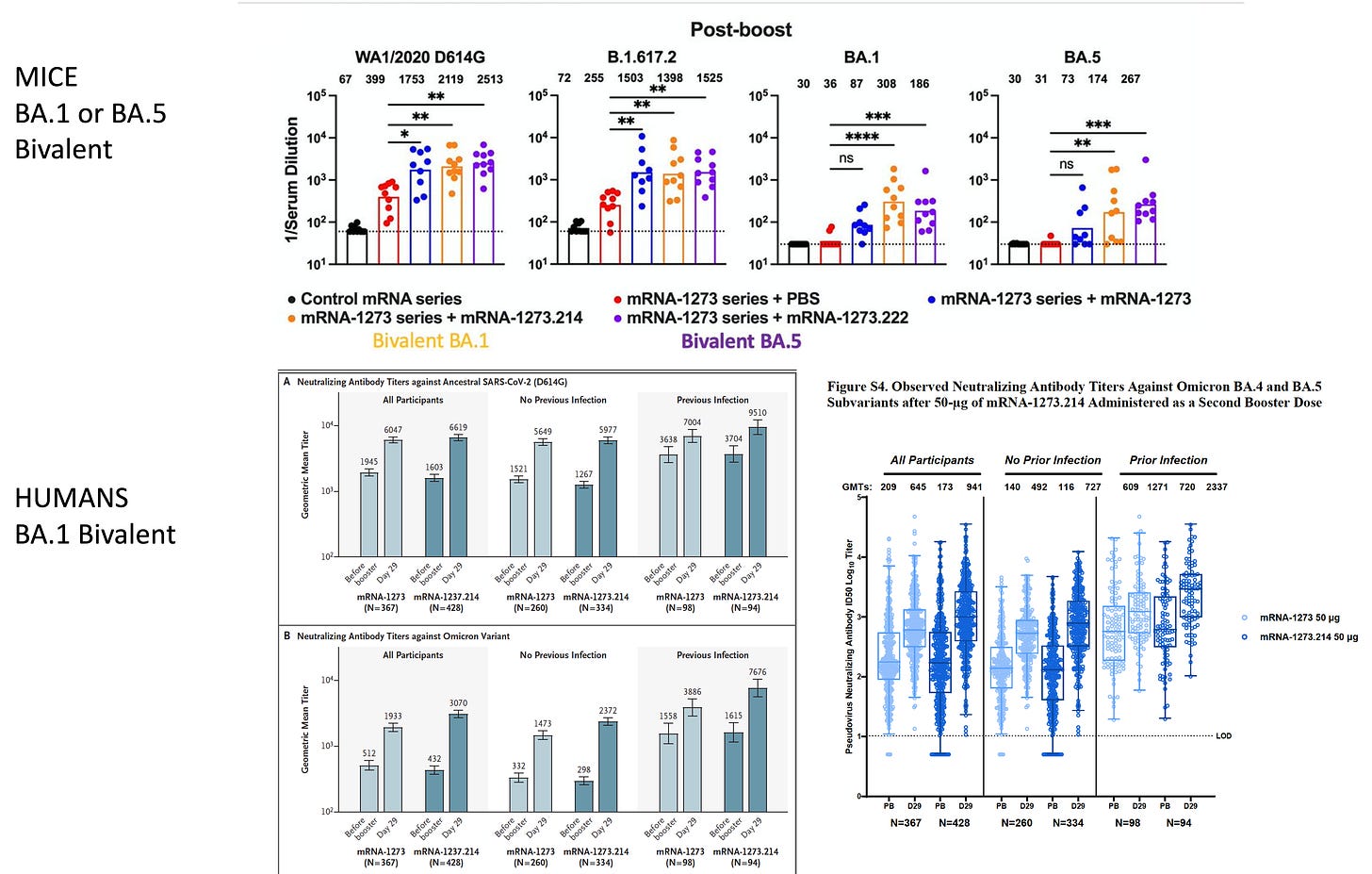
The Moderna BA.1 bivalent vaccine booster data were just published and showed about a doubling of neutralizing antibodies vs BA.1 (and BA.5) for this vaccine as compared with the original vaccine booster. That’s good but leaves the question as to whether the BA.5 bivalent will do any better. From both the human data from this report and the data from mice the same vaccine, there is a disturbing trend of much lower antibody induction vs Omicron compared to that mounted against the ancestral (original) strain. Here are the highlighted participant data from the report
And also the data from mice with the same vaccine booster, which are consistently showing a reduced level of antibody induction.
Why is this happening?
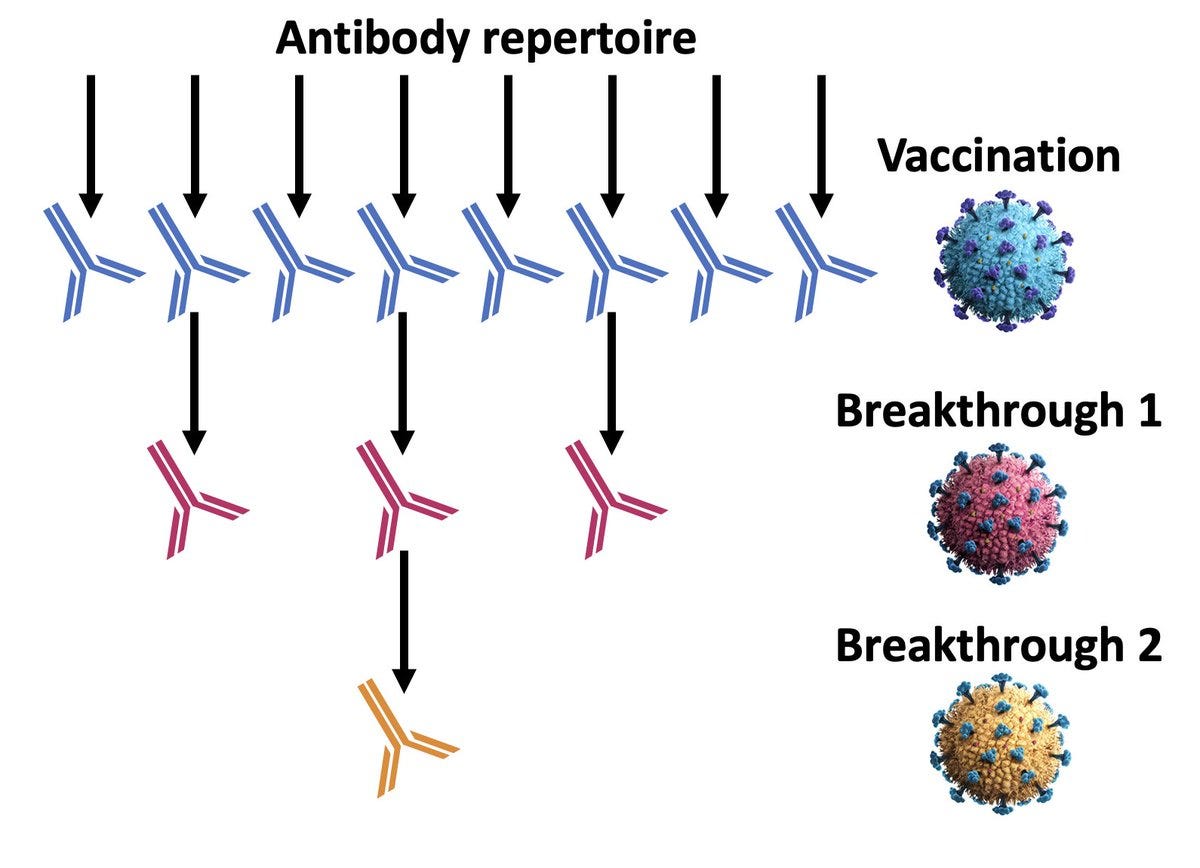
There are 2 possible explanations: that the Omicron variants are less immunogenic or there is imprinting, with a reduced capability of mounting a response to a new antigen because of being primed by the first exposure (be it infection or by the vaccine). This is, of course, a simplistic explanation because we’re just talking about levels of antibodies and not subtypes by epitopes, no less memory B and T cells. Nonetheless, the Figure from Ulrich Elling is apropos, capturing the issue of imprinting. Perhaps what we’re seeing here is a combination of the immune escape (less immunogenic) and imprinting. But it’s certainly a concern that there are not much higher levels of neutralizing antibodies, with this metric considered as a surrogate marker for protection vs severe Covid.
The concerns about the imprinting were an outgrowth and reinforced by a new report from Yunlong Cao and colleagues. There was a significant reduction of neutralizing antibody epitope (antigen) diversity, and more non-neutralizing antibodies seen with BA.5 breakthrough infections. And likewise, that would be anticipated with BA.5-specific vaccine boosters. We await the upcoming Pfizer and Moderna new booster data to see if this report has predicted the immunologic response to the BA.5 bivalent vaccine.
The right question
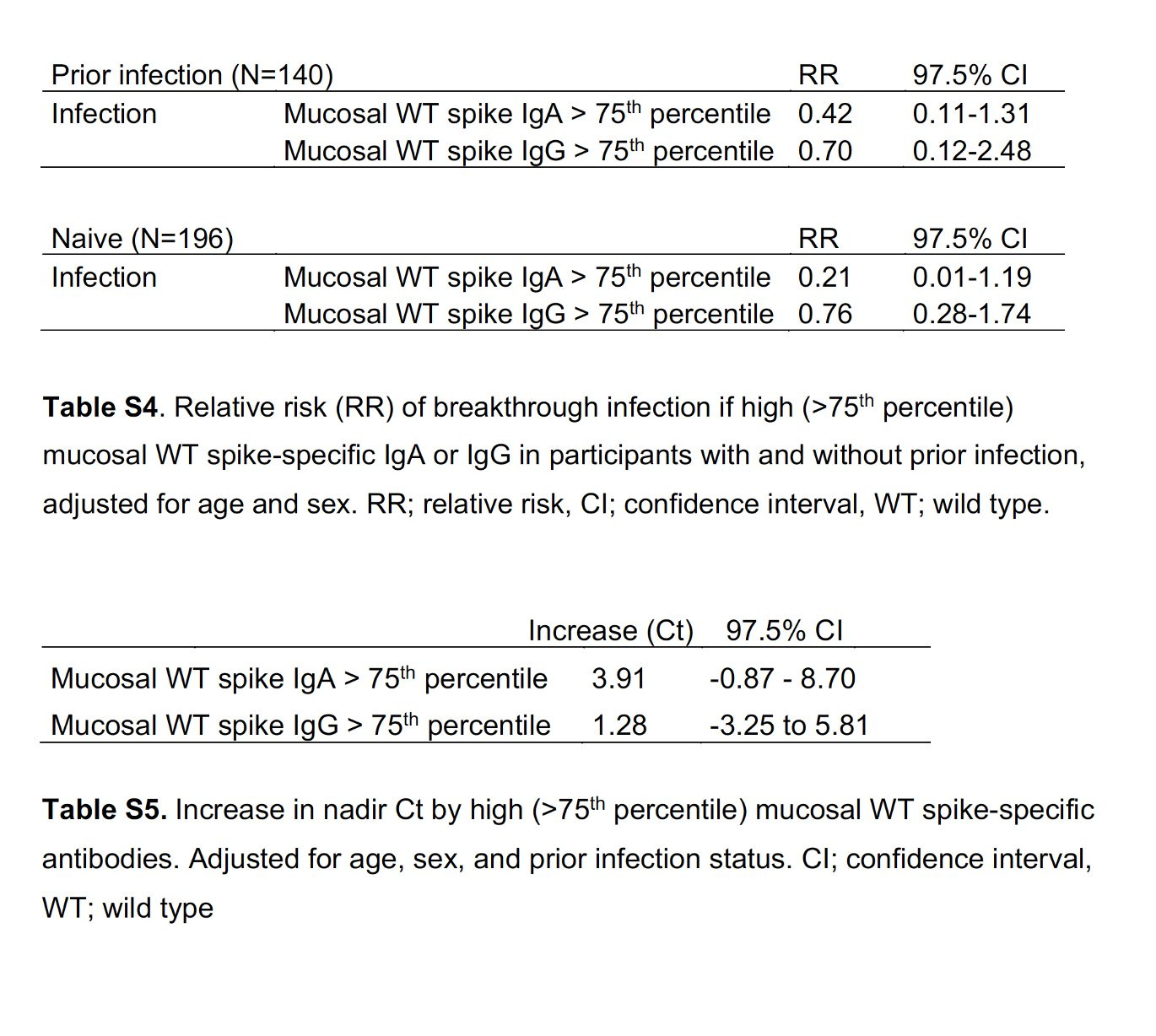
Boosters provide substantive and unequivocal benefit for protection from severe Covid and help reduce Long Covid (magnitude uncertain), and still, despite the challenges of Omicron, have some early (~2 months) effect for reducing infection and transmission. We don’t know yet if the BA.5 bivalent booster is any better than the BA.1 or the original booster. Based on the evolution of the virus through Omicron and its subvariants, it appears unlikely the new vaccine will have a major or important impact on reducing infection or transmission (we got a hint of that from the new BA.1 NEJM study above). There’s ample evidence from multiple studies that mucosal IgA antibodies are what will be needed to help block infections and transmission, such as this NEJM new report with 60-80% reduction of breakthrough infections (and reduced viral load, higher Ct, Tables below) as a function of mucosal IgA antibodies, not related to IgG antibodies. While they were formed in some health care workers as a response to vaccination and or infection, there is a way to induce them via nasal or oral vaccines. The durability of this effect isn’t yet known, but it would be far easier to take a nasal spray repetitively, with expectation of much less side effects, than shots. Certainly encouraging data from CanSino’s newly approved inhaled vaccine vs Omicron is a solid precursor for the many programs that are in advanced clinical trials.
The right question is about the future. We can’t go on getting boosters every 4 to 6 months and the premise of an “annual” shot is that the virus exhibits seasonality like flu, which certainly isn’t the case.
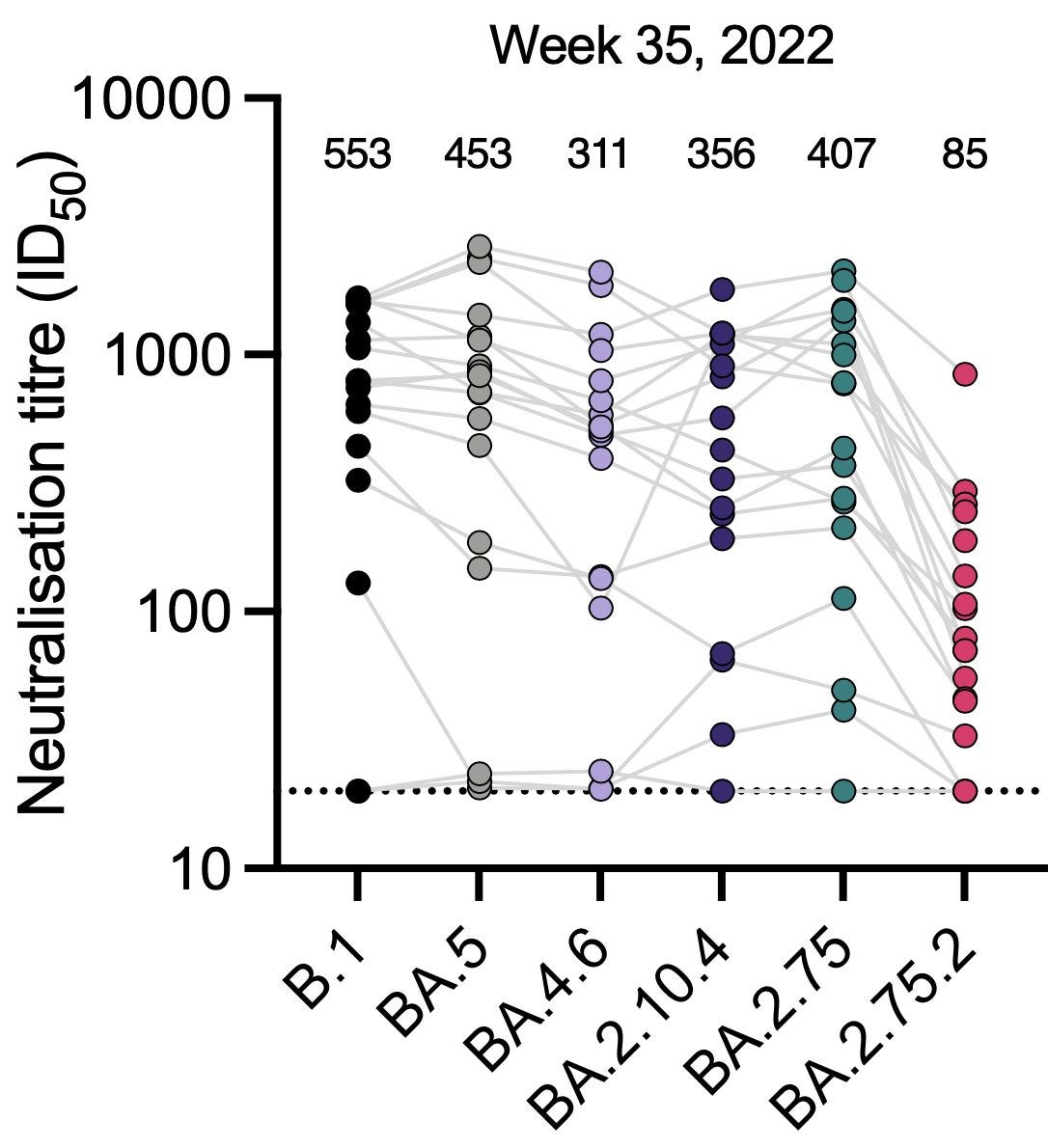
We have a new variant to be concerned about: BA.2.75.2, a daughter of BA.2.75 ,with three new spike mutations that are troubling. You can see its immune escape from the new preprint from Ben Murrell and colleagues. This variant has the most immune escape these investigators at the Karolinska Institute have yet seen, and that has been replicated by Yunlong Cao’s group in Peking. Given these observations, our current variant-chasing strategy to catch up to BA.5 will not likely help us counter BA.2.75.2. That underscores the need for variant-proof efforts.
In summary, there’s ample evidence that a 3rd shot or 4th shot (1st or 2nd booster) will help provide important protection, and that is especially vital for people age 50+, with ample support for the recommendation for all age 12 and older to get boosters. The right question is about the 5th booster, for which there are no clinical data yet, but will likely extend a high level of protection against severe Covid. But 4 or 6 months isn’t going to cut it as a public health protection policy, as there will be further attrition of interest and uptake for boosters as we go forward. Fortunately, we’re declining in cases and will likely experience a fairly quiescent phase (further descent, no surge) with respect to infections and hospitalizations for the next couple of months until BA.2.75.2 gets legs (or an alternative BA.2 derivative).
Now is the time to stop chasing SARS-CoV-2 and start mounting an aggressive get- ahead strategy. There’s the intertwined triad to contend with: more immune escape, more evidence of imprinting, and the inevitability of new variants that are already laying a foundation for spread. Enough of the booster after booster, shot-centric approach; it has been formidable, lifesaving, sickness-avoiding, and essential as a bootstrap, temporizing measure. Now we need to press on with innovation for more durable, palatable, and effective solutions. They are in our reach.