A Big Week for GLP-1 Drugs
A Breakthrough Award, New Evidence in Parkinson's and Heart Disease
There’s been no shortage of buzz about the GLP-1 family of drugs, but that was amplified in many ways this week. In conjunction with this edition of Ground Truths there’s a podcast with Daniel Drucker, the physician-scientist credited as one of the co-discovers of glucagon-like peptide (GLP-1) nearly 3 decades ago.(Sorry that you’re getting 2 emails from me today!) He helps explain some of the new findings that I’ll be going through here. Before getting into those, a notable award recognition related to GLP-1 drug expansion from diabetes to obesity.
The Bhaumik Breakthrough of the Year Award
The American Association for the Advancement in Science (AAAS) gives out an annual breakthrough award. Last year it was for standout contributions for NASA’s James Webb Space telescope. This year it went to 2 scientists—Lotte Bjerre Knudsen and Richard DiMarchi—the individuals who pushed to get these drugs to treat obesity. While the 3 researchers —Daniel Drucker, Joel Haebner and Jens Juul Holst —who discovered GLP-1 have been widely and appropriately recognized for this groundbreaking work, it was developed exclusively for the treatment of diabetes. That remained the case for nearly two decades. The interesting back story to this award was to find out who were the scientists that recognized and pushed for its potential use for obesity. I participated on the selection committee with John Bush, Katherine Saunders, and Holden Thorp. It wasn’t easy, and it required a lot of translation of things written in Danish and connecting with many key players in the field. Ultimately, Bjerre Knudsen of Novo Nordisk and Richard DiMarchi of Indiana Unviersity were verified as the individuals who shaped this critical expansion. As summarized in the award announcement, “Bjerre Knudsen and DiMarchi overcame significant obstacles in bringing these drugs to patients seeking to lose weight. Bjerre Knudsen, for example, figured out how to make the peptide underlying this drug class — an otherwise ephemeral substance — last long enough in the body to be a medication. DiMarchi transformed thinking about the hormones this class oftherapies should mimic, a critical part of their efficacy.”
Despite critics who doubted obesity was a disease at all, they relentlessly pursued this use case, well beyond being the first 2 to patent it. Were it not for their efforts we might not have this unprecedented treatment for obesity, which has now unlocked many other potential clinical applications. That’s what we’ll get into now.
GLP-1 Drugs for Parkinson’s Disease
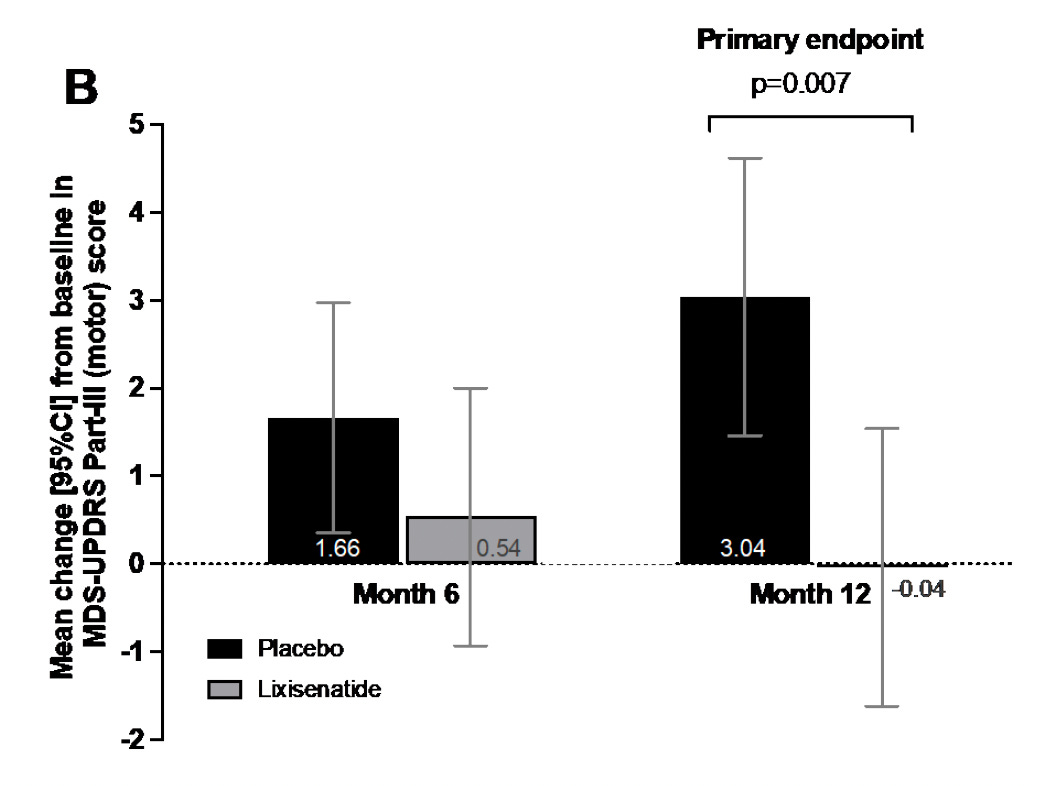
It’s important to first point out that there are no disease-modifying interventions for Parkinson’s disease. All current treatments are for symptoms but nothing has yet been validated to stop or slow progression of the disease. In the New England Journal of Medicine a double-blind randomized trial of 156 participants with early Parkinson’s disease were assigned to lixisenatide or placebo. Lixisentaide is a GLP-1 drug that was approved in the United States in 2016 and discontinued by its manufacturer (Sanofi) in 2023. The drug or matching placebo were given for 12 months and the primary endpoint, as shown below, was a motor score used by neurologists (Movement Disorder Scale-Unified Parkinson’s Disease Rating Scale) MDS-UPDRS Part III). The GLP-1 drug participants had no progression of motor symptoms (-0.04), and the 3.04 worsening in the placebo group (difference between the two arms=3.08) is just short of what is considered clinically meaningful (3.5).
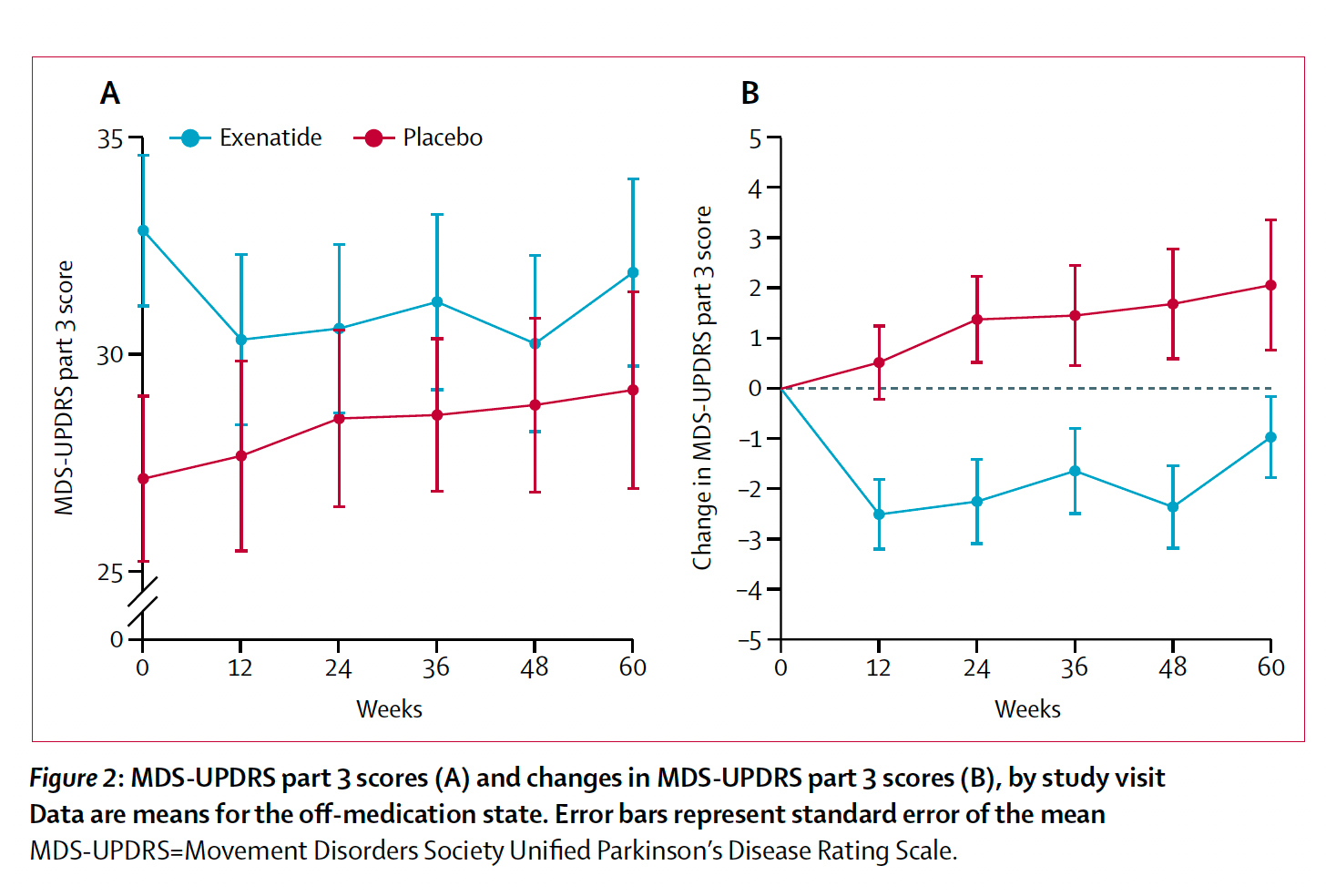
This is not the first randomized trial reporting benefit of GLP-1 drugs for Parkinson’s. In 2013, a single-blind trial of 45 patients with exenatide showed significant statistical benefit but the motor score difference was 2.7 points.
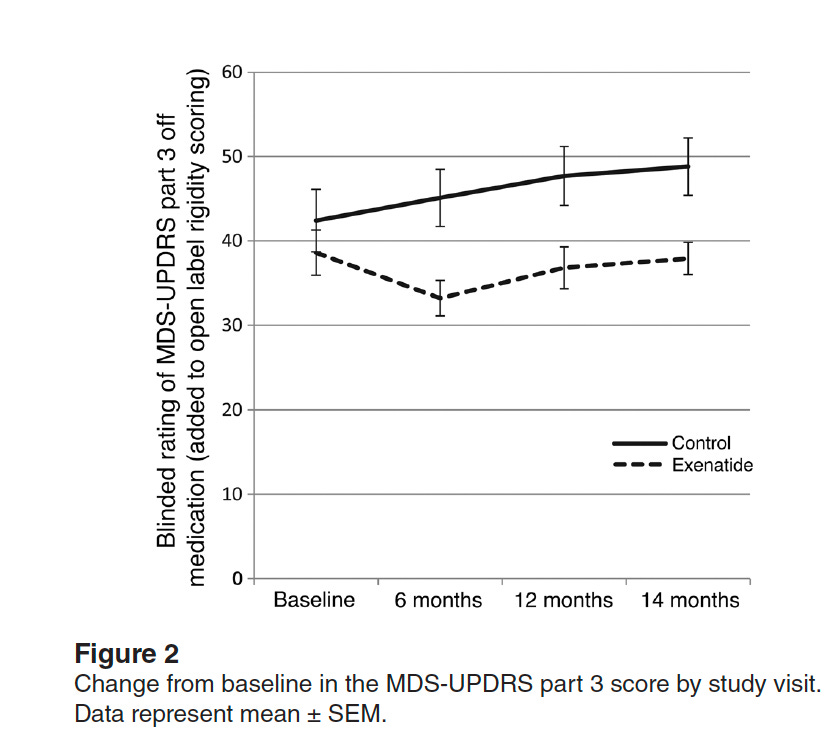
In 2017, a small single-center randomized trial of 62 participants, but with longer follow-up, found benefit with exenatide given once weekly (see graph below). It concluded: "There is a strong indication that GLP-1 receptor agonists may have a useful role in future treatment of Parkinson's disease."
Of note, both trials had a washout period after the drug treatment ended and the benefit for motor symptoms persisted.
A key point made into accompanying editorial for the new trial is that the >3-point benefit may accrue further over time: “The importance of this finding is not the magnitude of the change but what it portends….if the benefit of lixisenatide is cumulative, adding another three points each year over a period of 5 to 10 years or more, then this could be a trill transformative treatment.”
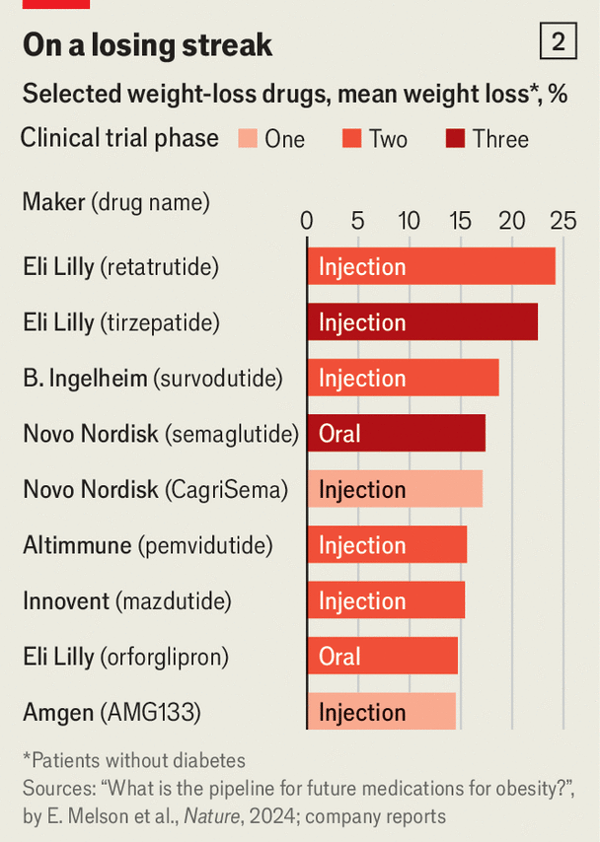
There are 2 other important considerations. Lixisenatide and exenatide are much weaker GLP-1 drugs compared with semaglutide, tirzepatide and several others that are in clinical trials as shown below for weight loss. The extent of weight loss likely also reflects the potency of anti-inflammatory effect, and biomarkers indicative of reduced inflammation have been shown to precede wight loss in multiple trials of this drug class.
Also, we know that these drugs do not cross the blood brain barrier. As Daniel Drucker explained in the companion podcast, that isn’t necessary to achieve the GLP-1 mediated suppression of inflammation in the brain. There’s the gut-brain axis and enteric nervous system to relay signals, talk to the brain. As Drucker pointed out to me: “ We know that GLP-1 is not getting directly to those neurons, but it's activating pathways that turn on those neurons. And so, there's probably a very intricate set of pathways that sense the GLP-1 and the accessible neurons and then transmit those signals deeper into the brain.”
So the new study provides some optimism for Parkinson’s disease. The GLP-1 drug mediated suppression of brain inflammation may turn out to be yet another key use case. Note there are 2 large randomized trials of semaglutide for Alzheimer’s disease expected to report out in 2026.
The Benefit For Heart Failure With Preserved Ejection in a New Trial
More than half of patients with heart failure have preserved pump function, as quantified by election fraction. Their heart failure symptoms and physical limitations are due to impaired filling and relaxation of the left ventricle.
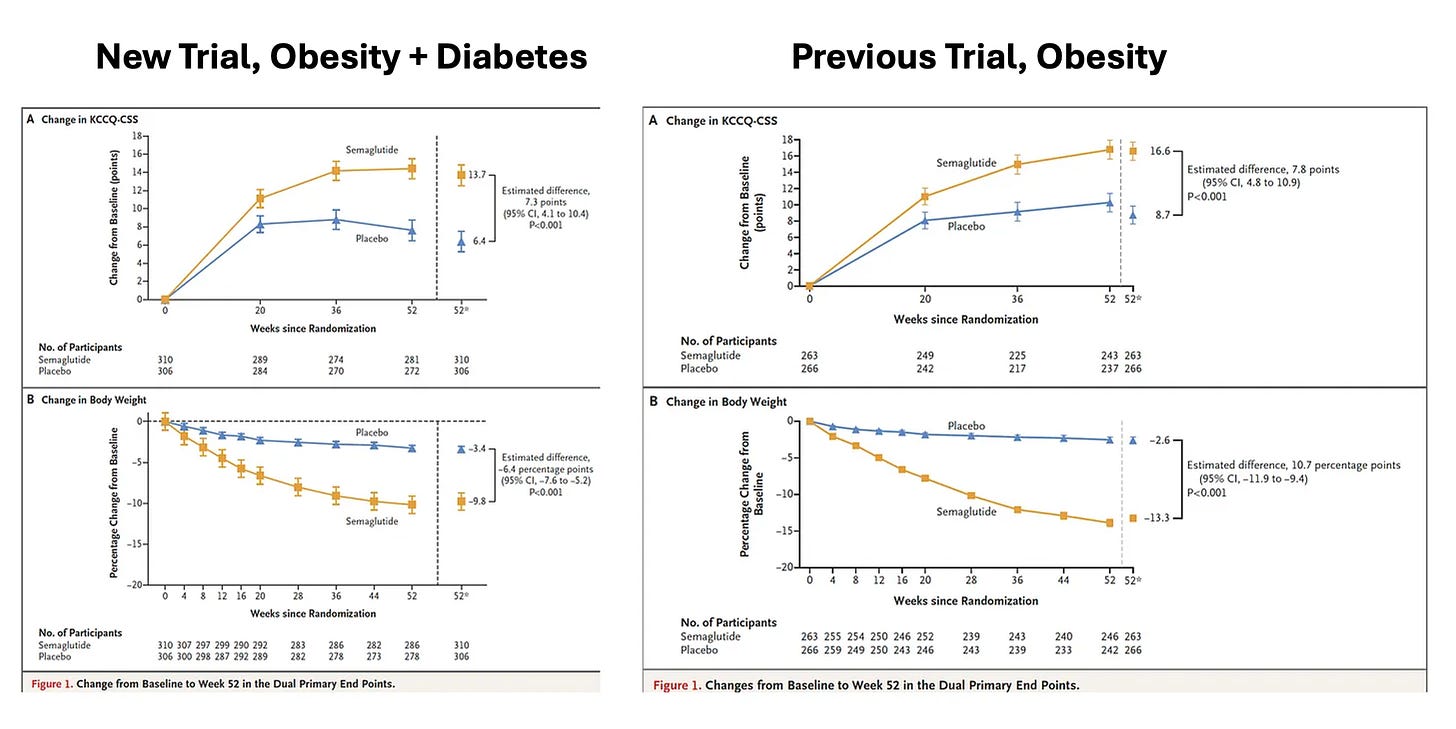
At the American College of Cardiology meetings today, with simultaneous publication in NEJM, was a new randomized trial of semaglutide in 616 participants with heart failure with preserved ejection fraction, obesity and Type 2 diabetes. This new trial built on a previous one published in NEJM last fall (I previously reviewed here) for the same indication, but the only difference was the inclusion of people with diabetes. As you can see from the side-by-side Figure below, the new trial (at left) replicates the previous one for symptomatic benefit and reduced physical limitations (Y-axis, corresponding to the Kansas City score) with the primary endpoint findings remarkably similar. The new trial thus strongly reinforces the benefit of GLP-1 for heart failure with preserved ejection fraction and extends that to people with Type 2 diabetes.
There’s a big footnote for this trial that takes us back to the Bhaumik award and why there was such a long time before the realization the GLP-1 drugs could be useful for obesity—people with diabetes on GLP-1 drugs didn’t lose much weight. If you look closely at the bottom panels of the 2 graphs above for loss of body weight, the participants in the new trial had 40% less weight loss than in the previous trial of obese, non-diabetic participants. We still don’t know why people with diabetes (and obesity) don’t lose as much weight compared with obesity without diabetes (as Drucker and I discussed in the companion podcast). The new trial tells us that the mechanism of heart benefit is not only dependent on weight loss since a similar magnitude of symptomatic relief was seen in the 2 trials.
Glucose Lowering Medications for Patients With Type 2 Diabetes
A large retrospective study of medication used in diabetes and outcomes was also published this week. About 40-50,000 patients receive GLP-1 receptor agonists or sodium-glucose cotransporter-2 inhibitors(SGLT-2i) drugs, 84,000 were treated with dipeptidyl peptidase-4 (DPP-4) and more than 200,000 received sulfonylureas, with tracking of major adverse outcomes of all-cause mortality, heart attack, stroke, need for coronary revascularization. The bottom line: “Our findings suggest that GLP-1RA and SGLT2i (and especially SGLT2i with the lowest number needed to treat (NNT) for nearly all study outcomes) may be the preferred glucose-lowering agents for cardiovascular risk reduction in patients at moderate risk for CVD, building on and consistent with existing RCT evidence in the high-risk population.” This report highlights potential preference for GLP-1 drugs as a first-line treatment for Type 2 diabetes related to its cardioprotective effect. The potential additive protective benefit of GLP-1 and SGLT-2 drugs is intriguing but has not yet been explored in randomized trials.
Summary
Pulling the new reports together, there is further momentum and excitement building for GLP-1 drug expanded use beyond diabetes and obesity. The treatment benefit for heart failure with preserved ejection fraction, accounting for more than half of heart failure cases, has now been replicated. The key finding of much less weight loss in the new trial with Type 2 diabetes helps us understand the story of GLP-1’s success— it’s not just because of reduced body weight. Especially of interest are the new findings in Parkinson’s disease. We desperately need an intervention that is disease-modifying and now there’s a chance we’ll get one. That will require a larger trial with longer duration of treatment—it deserves a high priority given the current results. With the effects seen of the GLP-1 family of drugs on preventing kidney damage in diabetes, liver disease (non-alcoholic or metabolic-associated steatosis, NASH/MASH), and addiction, this drug class is exceeding all expectations. Please consider taking time to listen to (or read the transcript) of the companion podcast with Daniel Drucker to get insights from his expertise, now >30 years of studying GLP-1 and related drugs.
Thanks for subscribing to Ground Truths!
The Ground Truths newsletters and podcasts are all free, open-access.
Voluntary paid subscriptions all go to support Scripps Research.



Hi Dr. T: Thanks for your great work. Can you speculate on how GLP-1 medications impact Parkinson’s when they don’t cross the blood brain barrier? Are there any other connections between, say, gut biome neurology, and extrapyramidal function? Thanks- Greg Sazima, MD
The shortage of these medications is getting pretty intense, with many of my patients scrambling to find refills and many having to stop cold turkey. The good clinical news keeps coming, and I believe the theoretical increased risk of pancreatic cancers has not materialized. Still waiting on definitive news about thyroid tumors, though this also seems unlikely outside of rat trials.
Some serious GI side effects for many, but also some serious success stories for many more.
Bravo indeed to these individuals, as these meds are now a commonplace in primary care treatment repertoires.