Steve Horvath made the seminal discovery of the—Horvath Clock— an epigenetic clock based on DNA methylation, which is now being used extensively in medical research and offered commercially for individuals (←we talk about that!). He was on the faculty at UCLA from 2000-2022 as a Professor of Human Genetics and Biostatistics, and now works on anti-aging research at Altos Labs.
A perspective on the importance of epigenetic clocks this week’s Nature
”This insight is crucial for deriving reliable biological markers of ageing in tissues or blood. Such a feat has been accomplished through the ingenious identification of epigenetic clocks in our genome. But these insights are even more important for revealing targets that enable intervention in the ageing process.”
A video snippet on vegetable intake and epigenetic clocks. Full videos of all Ground Truths podcasts can be seen on YouTube here. The audios are also available on Apple and Spotify.
Transcript with links to Audio and External Links
Eric Topol (00:06):
Hello, it's Eric Topol with Ground Truths, and I've got a terrific guest with me today, Steve Horvath. He's a geneticist, a statistician, a mathematician. He's got a lot of background that has led to what is a landmark finding in biomedicine, the Horvath clock. So Steve, welcome.
Steve Horvath (00:30):
Thank you for having me.
Eric Topol (00:33):
Well, it's really fascinating. I followed your work for well over a decade since you introduced the pan-tissue clock in 2013, and it's fascinating to go back a bit on that finding, which initially, I guess was in saliva a couple of years prior, and then you found it everywhere you looked, wherever cells had a nucleus and tissues. And what gave you the sense that these markers of methylation on the DNA would give us some clues about the aging process? How did you even come about to make this discovery?
Serendipity
Steve Horvath (01:17):
It was an accidental discovery because before the methylation clock, I had worked very hard on a gene expression clock, a transcriptomic biomarker. I mean, I was at the height of my energy levels. I worked really on weekends, really eight hour days during the week. But all the weekends I had collected a large set of gene expression data and I dredged the data. And for two years and I couldn't get anywhere, there was nothing I could do. But nowadays, of course, you see various publications where people built transcriptomic clocks. But back in the day when we had these arrays, I just couldn't see a signal. And then at some point I got roped into a study of homosexuality where my collaborator at UCLA wanted to see whether there's an epigenetic correlate of sexual orientation in saliva. And so yeah, being a biostatistician, I said, sure, I analyzed the data and I couldn't find any signal for homosexuality.
(02:48):
But then I just looked for an aging signal in the same, and really within an hour of analyzing the data, I knew that I have to completely drop gene expression. I need to go after methylation. And the signal is so profound, and as you said initially we looked at saliva samples and we thought, isn't it curious? You spit in a cup and you can measure someone's age. And we were of course, hoping that this could become a valuable readout of biologic age, but it took, of course, many years to realize that potential. Nowadays, there's several companies that offer a saliva based methylation clock test. But yeah, many years passed, and it was important to fill in the details and to build the case that methylation clocks are predictive of things we care about time to death or time to various forms of morbidity. So it took many, many years to analyze large cohort studies and to accumulate the evidence that it actually works.
Eric Topol (04:16):
Yeah, I mean, it was pretty amazing back almost a decade ago when I would see, we would take tissue or blood sample and look at your clock and it would say, age of the person is 75 years. And then we look at the actual age of the person who is 75 years to say, wait a minute, how can this be? So I mean, the plausibility of this discovery, if you look back, I mean you say, well, this is just kind of the rust of the pipes, or how do you process that the methylation is such a marker potentially of a person's biologic age? Of course, we're going to get into how it could be a way to intervene to change the aging process. But would it be fair to say that its epigenetic clocks are not the same as biologic aging or how do you put all that together?
Epigenetic Age vs Biologic Age
Steve Horvath (05:21):
Yes, for sure. An epigenetic age estimate is certainly not the same as a biologic age estimate. And the reason why I say it is because biologic age is really determined by so many things and by so many organs. And as I mentioned initially, we had a clock for saliva later for blood and so on. And so, if you only have an epigenetic readout of a certain cell type, it's really too limited to assess the whole organismal state. And arguably you would want to measure also proteomics, readouts and many other data modalities. So I typically avoid the terminology biologic age, because to begin with, we don't have a definition of it. Decades of discussions, nobody really has a precise definition of it.
Second Generation Epigenetic Clocks
Eric Topol (06:35):
Well, from the first generation Horvath clock then became this newer second generation, GrimAge, PhenoAge, the DunedinPACE of aging. How has that helped to advance the field? Because as you touched on, they're measuring different things and what is it meant by kind of a second generation clock?
Steve Horvath (07:03):
Yeah, so a second generation clock truly aims to predict mortality or morbidity risk. As opposed to simply chronologic age or what is known as calendar age. And fortunately, there's no doubt that the second generation clocks can do that. I often finish a talk on GrimAge by telling the audience that I give them a money back guarantee, that it will be predictive of mortality in their cohort study. I'm 100% certain that it works if you analyze a hundred people or so. The question is more whether an individual could benefit from such a test. And there are now many providers of various epigenetic clock tests. These biomarkers have different names, but they're quite pricey. A couple of hundred dollars are needed to get such a measurement. And the question is, is it helpful for the individual should you get such a test? And I would say we are not quite there yet for a variety of reasons. The main reason being we don't have good interventions against accelerated epigenetic age. So because when you think about it, why does a doctor order a test for you? For example, cholesterol levels. Well, because they have a drug against elevated cholesterol levels, the statin. And at the moment, we don't have validated interventions against accelerated epigenetic age. So that's kind of missing.
Eric Topol (09:13):
Yeah, we're going to get to that because obviously a lot of things are in the pipeline there, but are you saying then that these people that are getting these consumer tests, that they're getting a test that really wasn't validated at an individual level, so it predicts their mortality that it may be good at a cohort or population level, but maybe it's not so helpful, accurate, or would you say it is accurate? I mean, GrimAge is a good name because since it says when you're going to die. How do you make the differentiation between the individual level or beyond?
Steve Horvath (09:59):
Yeah, I think it's good to compare to other biomarkers. So take glucose levels, hemoglobin A1C, nobody doubts that these levels predict mortality risk when you study couples a hundred people. But how accurate is such a test for an individual? Clearly there is substantial noise associated with a prediction. Two people could have exactly the same hemoglobin A1C levels, but live very different lifespans. And the same holds for epigenetic clocks. They do predict how long you live. In theory, one could arrive at an estimate of age and death. There's a complicated mathematical formula that allows you to do that, but there would be a substantial error bar associated with it, an order of magnitude plus minus five years. And so, for the individual, such an estimate is not that important because the error bar is substantial. But I want to add that these second generation clocks, they do predict mortality risk. There's no question.
Maximal Lifespan
Eric Topol (11:35):
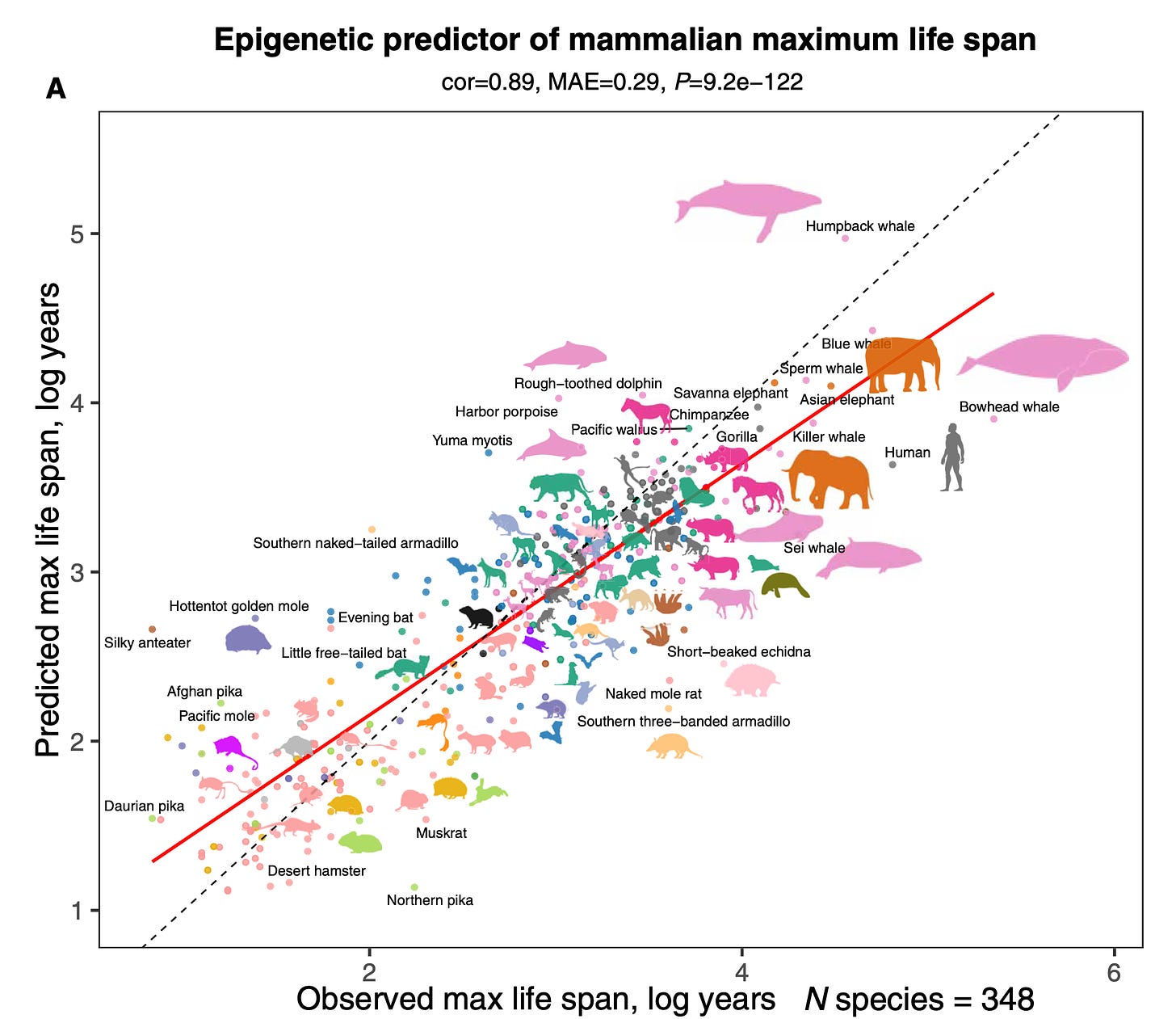
Well, as you know, the longevity space is now very crowded with all sorts of clubs, and it's like a circus out there. And some of these things are being promoted that really don't have the basis or have a false sense to consumers who want to live forever and be healthy forever. But maybe these markers are not really helping guide them so much. Now, you recently published you and your group a fascinating paper, so getting away from the individual for a second, but now at the species level and in Science Advances, and we'll put this diagram with the podcast, but you looked at 348 mammal species for the maximal lifespan with DNA methylation. And it was amazing to see the display from the desert hamster all the way to the humpback whale with somewhere along the way, the humans. So you could predict maximal lifespan pretty well, right?
Steve Horvath (12:43):
Yes. So I collected this very large dataset over seven years, and one of the reasons was to understand the mystery of maximum lifespan. The bowhead whale can live over 211 years, whereas certain mice only three or four years. And my question was, can methylation teach us something about maximum lifespan? And the answer is a resounding, yes. The methylation profiles very much predict the maximum lifespan of a species. And maybe to use a metaphor to explain the patterns. So one can visualize methylation around the DNA molecule, like a landscape. You want that certain regions exhibit high levels of methylation. These regions must be really shut down and other parts of the DNA as opposed to exhibit very low methylation, for example, a transcriptional start sites. And long lived species have a very hilly landscapes, high hills of methylation and steep valleys of low methylation. Where shorter lived species have flatter landscapes. So that was one of the insights of that study. The other perhaps paradoxical insight was that the locations in our DNA that gain methylation with chronologic age, these regions often differ from regions that determine the maximum lifespan of our species. So that's a bit perhaps paradoxical and counterintuitive, but it just shows that the DNA encodes our species characteristics at different locations from our mortality risk.
The Other Clocks
Eric Topol (15:13):
Right. No, and I mean it's fascinating. I can imagine how it could take seven years to pull all that data together. It's amazing. Now, one of the issues of course, is if you're trying to gauge the biologic age, which we already established is somewhat different than epigenetic age or a clock, there are many different ways to do that. And you mentioned transcriptome clocks, which are not as well perhaps developed. Obviously, none of these others are developed like the Horvath clock and newer generation clocks, but there's immuno aging clocks like iAge, there's proteomic clocks, there's organ clocks with high-throughput proteomics, thousands of proteins. Do you see these as complimentary, like orthogonal where they each add to the story? Or do you really see the methylation as distinct?
Steve Horvath (16:20):
Well, I think ideally you measure all of the above to really get a very granular understanding of different facets of aging. And however, scientists always like to find deep connections between different readouts. For example, it would be wonderful if we could use proteomics instead of methylation, or my group has worked on the opposite. So we can actually estimate protein levels in the plasma based on methylation for about 10% of all plasma proteins, you can estimate their levels based on methylation. So yeah, people who are interested in these deeper programs that ideally link everything, some sort of aging program that underlies these different manifestations of aging, they will want to reduce everything. But until we have a deeper understanding, I think let's air on the side of measuring too much.
Eric Topol (17:45):
Well, what's interesting, as you mentioned, I didn't realize you could basically impute the protein story from the methylation, but one of the issues is if you want to do 11,000 plasma proteins, it could cost a thousand dollars. But if you want to do a bisulfite methylation, you might do that for very inexpensively. So there's a practical part of this too, and the immune characterization is even more expensive and difficult from a practical standpoint. So we go back to that initial work that you did and how you got into an area that is practical, inexpensive compared to some of the alternatives. But as you say, they may have features that are also helpful. Now, this is now the craze, this epigenetic clocks, and I want to mention you probably didn't see it because it's not a journal that you would look at, but just yesterday, July 29th, there were 12 papers published in JAMA Network Open.
Modulating Your Epigenetic Clock
(18:51):
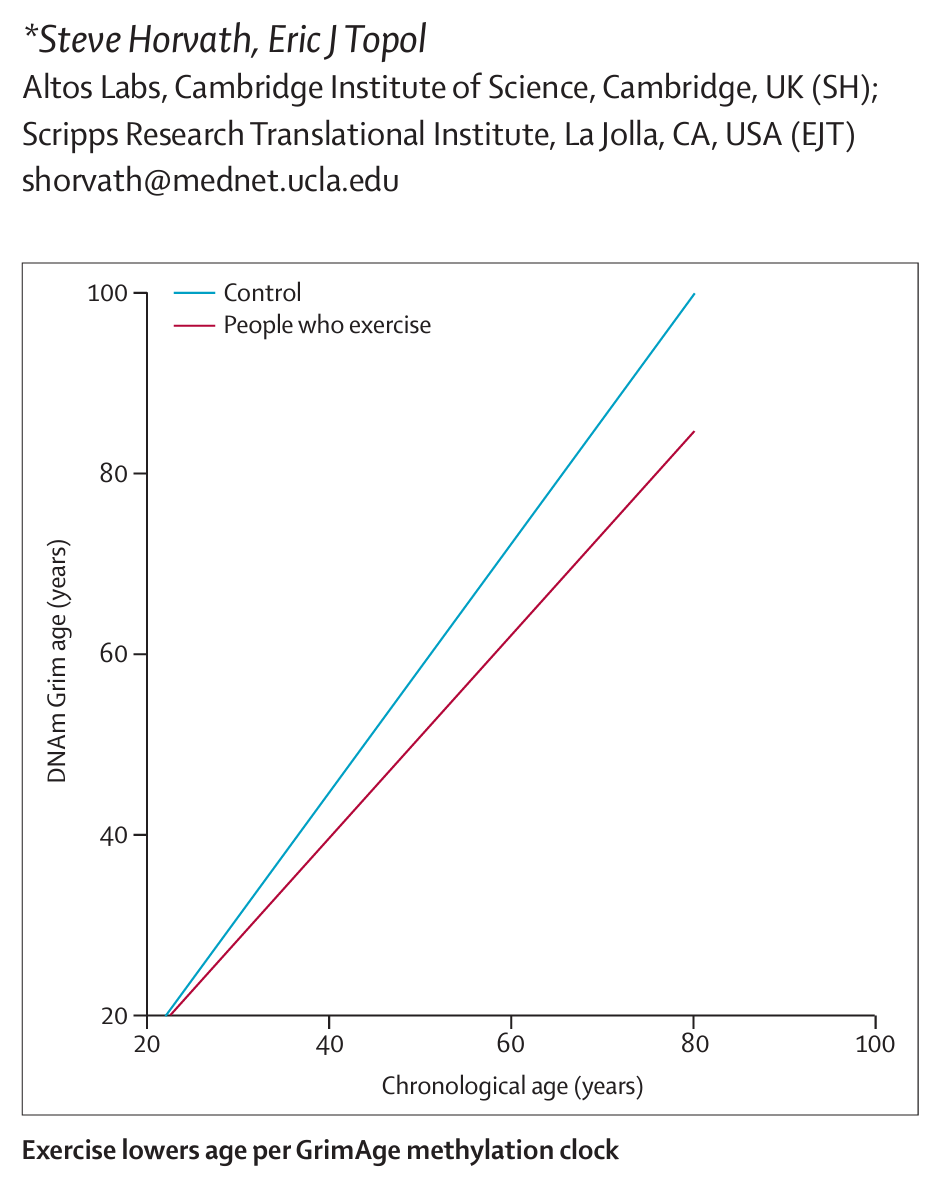
Everything from how loss of loved ones changes your epigenetic clock to PTSD, to vegan diets, to inequities. I mean, just incredible. So it is the rage now. It's taken the biomedical community some years to catch up to where you were. And one of the things of course that we know that from your prior work that is an intervention that helps give a less accelerated epigenetic clock is exercise. And in fact, that was highlighted in our Lancet essay in the first week August issue. But can you comment on that and anything else that we know like plant diets and anything that favorably influence our DNA methylation pattern?
Steve Horvath (19:52):
Yes. So interestingly, vegetable intake really has a strong effect on GrimAge and many other epigenetic clocks. And maybe this is obvious to the listener, everybody knows that vegetable intake is healthy. However, it's very surprising to me as a scientist to contemplate how is it that vegetable intake affects the methylation levels of your blood? How does it affect the hematopoietic stem cells? I just don't understand the mechanism behind it, and however, the effect is very strong. So we studied postmenopausal women in the women's health initiative, and for these women, we had blood measures of carotenoid levels. So this is an objective measure of vegetable intake, and the correlations were substantial. So that's one intervention I'm quite certain about. Other intervention that have a strong effect relate to metabolic syndrome, anything that relates to type 2 diabetes such as obesity, high glucose levels, that part of the biology very much affects our epigenetic clocks. So disturbed metabolism has a strong effect.
Eric Topol (21:37):
Has these findings changed your diet or made you exercise more or anything like that?
Steve Horvath (21:44):
. So I eat a lot of frozen vegetables. My freezer as full frozen vegetables.
Eric Topol (21:56):
That's great. Well, there's a lot of uses today as we touched on in the Lancet piece as we're waiting for more benchmarking and more work on this. But for example, we have a shortage of donor organs, and there are people who might be of calendar age advanced, but their epigenetic clock might put them at a much younger age. Is that ready for use in the transplant world as one application?
Steve Horvath (22:37):
I haven't seen that yet. I've seen several studies that have explored that idea. The idea is rather obvious, but I haven't seen it implemented in practicum.
Eric Topol (22:53):
Another one is that we don't, as you've seen from some of these studies on organ clocks, our organs age at different paces and some people are accelerated heart agers or brain agers. If you had access to tissue to get methylation, would you see the same thing or this is of course of interest because we're trying to understand high risk individuals for age related diseases, whether it's dementia or heart disease or cancer. So is the second generation clocks like PhenoAge just good enough, or would you think that the organ clocks would give you some added insight?
Steve Horvath (23:47):
Yeah, I would say this is literally the frontier of research. Several groups attempt to use blood methylation or saliva or skin or fat adipose as surrogates for various other organs. And I've seen very encouraging results. So I do think this idea makes scientific sense, and which comes back to one of the miracles of methylation that this is even possible because if you had written a grant 10 years ago where you said, I will measure blood methylation to assess cognitive functioning, for example, you wouldn't have received any score, not in no funding, but however, interestingly, blood methylation does relate to cognitive functioning and many other organ functions. And so, the proof of concepts have been established. Blood methylation relates to fatty liver disease, kidney disease, lung disease. It has all been done in epidemiological studies. However, the question is how much could a blood methylation measurement help an individual? Should I measure my blood methylation to learn about my liver? And I would say we are not there yet because arguably there are wonderful plasma biomarkers to assess organ functions. And in certain ways, one needs to provide evidence that a methylation measurement is superior or compliments plasma based biomarker. And that's a hard hurdle to take.
Eric Topol (26:02):
Right. I imagine someday it may become the norm of assessing people's risk, but as you say, we're not there yet because it's a tough bar to meet, for sure. Now, you were a Professor from year 2000 at UCLA in multiple departments in genetics and biostats, and then in more recent times you joined the Cambridge unit of Altos, which is one of the companies that has gotten the most attention for its diverse efforts towards modulating, rejuvenating the aging process. So you and many top scientists around the world were recruited to Altos. I know some here at the San Diego campus. Was this thinking that it could help accelerate the whole idea of modulating aging in a favorably way or where do you see that the biotech world can play a role?
Can We Change the Pace of Aging?
Steve Horvath (27:15):
Yes. I mean, speaking for myself, I was getting tired of writing scientific papers and not affecting clinical care. I felt I needed to help identify or validate rejuvenating interventions because of the great promise, and this is perhaps best done in the setting of a biotech that is focused on translation. And that's why I joined. I'm moving away from biomarker development towards finding interventions that move the needle and ideally rejuvenate multiple organs and cell types at the same time.
Eric Topol (28:09):
Right. Now, there's lots of ideas of how we could do that from senolytics that would get rid of specific senescent cells that are bad actors to epigenetic reprogramming or chemical reprogramming or so many anti-inflammatory, like the recent paper of IL-11 that I'm sure you saw in Nature just a couple of weeks ago and many, many other ways to get there. What are you thinking? Is this going to be possible? Obviously, there's lots of naysayers. Is it going to be possible body wide or only for specific ways? For example, maybe we could bring back the thymus from its involution or we could stop ovarian failure in women so that their loss of advantage is delayed many years. Or do you think we're going to get to body wide anti-aging?
Steve Horvath (29:13):
Yeah, I think of it as divide and conquer. So ultimately I do believe that we can rejuvenate most cell types and tissues. The question is how do you roll out this program? Do you look for this one silver bullet that does it? For example, this idea of interrupted reprogramming based on Yamanaka factor combinations that looks of course very promising and rodent models. But then such silver bullet treatments could be risky for patient keyword malignant transformation, cancer risk, and it could be far safer to focus on one organ system or one tissue. For example, David Sinclair's company Life Biosciences looks at optic nerve regeneration for a reason. It could be safer. And so yeah, I'm very happy that companies explore different strategies. Certain companies focus on one condition, fatty liver disease or NASH. Other companies focus on immune system restoration. But I think many people think of one condition as really a first step to establish safety and efficacy, and then hopefully they could translate it to other body systems and organ systems.
Eric Topol (31:02):
But is it fair to say you're optimistic that we will be able to change the aging pace in people?
Steve Horvath (31:10):
Yes, I think yes. I'm very optimistic and there are several reasons for this optimism. The first is that dramatic results can be achieved in mice and rats. So we and others have published studies that show that you can reduce the epigenetic age by 30% or so and you can extend the lifespan, and you cited this very exciting paper by Stuart Cook on IL-11 inhibition that just came out in Nature. So I keep seeing these kinds of headlines, and then I want to think that one of these will actually work for humans. So the second thing that makes me optimistic is really this combination of artificial intelligence and biomedical research. Then going forward, robotics. So I can see several ways of accelerating biomedical research. So I'm quite optimistic.
The Role of A.I.
Eric Topol (32:24):
Maybe go a little deeper on the AI potential to help here. How does AI come into play?
Steve Horvath (32:33):
So AI can help in so many different ways. The first topic is biomarker development. I of course spent 10 years on a certain statistical model for building biomarkers, which is known as penalized regression. It works well, but AI allows the community to build imaging based biomarkers. So for example, based on MRI images, but also cells growing in a dish, we can say this treatment aged the cells growing in the dish or rejuvenated them. So that's one topic, biomarker discovery. The second is, of course, to design small molecules, keyword, these protein design where it has greatly accelerated drug discovery. And there are several companies working in this space, and again, there's wonderful case studies that look very convincing to me. And the third aspect of AI is another obvious one. AI can read many papers. I mean, you could be a 50-year-old professor who has read papers their entire life, but an AI can really read far better and summarize insights better.
Eric Topol (34:27):
Yeah, the complimentary in terms of the reasoning of that information. So absolutely right now, one of the problems we have here is that aging is not seen as a disease. Of course, we can remember when obesity was not considered a disease and then there was a drug and everything changed. But here we don't have a classification it's a disease. It's considered a natural process that is highly variable in people. But the question is, we can't do studies that are going to wait 20, 30 years to find out if we promoted health span and lifespan. And so, we have to rely on these clocks. So how do you see this playing out? Do you think that we might see a regulatory approval on a surrogate proxy, like an advanced Horvath clock, or do you think that's not going to cut it, that you're going to have to show more to get a anti-aging treatment across the regulatory threshold?
Steve Horvath (35:42):
Yeah, that's a very good question. So I believe that the biomarker community has already assembled enough evidence to offer a battery of tests that could be used as surrogate endpoints of interventional study. And we could discuss the components of this battery. But I would say we already have biomarkers beyond just methylation. One could have the readouts of walking speed or muscle function, many readouts, and they could be aggregated into an index to summarize the biologic age, perhaps, of the individual. So that already exists. At the same time, this field is undergoing explosive growth. You mentioned every day new papers come out in the relatively small field of epigenetic clocks. There's so many papers that it's hard to keep track, but I embrace it. I think it's wonderful because clocks get ever more powerful.
(37:11):
So yeah, I would say there should be different versions. Ideally, a regulatory agency would make an executive decision and say, for the next three years, use the following five biomarkers. Then a few years later, as the science advances, they could come up with an updated version, but even a 90% solution would very much accelerate progress in the whole field of rejuvenating interventions. So I would very much embrace a top down decision on which biomarkers should be made, because the bottom up approach, by the way, simply doesn't work. The minute you put three professors in the room to come up with a decision, which biomarker is best, there will be three different opinions. We need impartial arbiter that makes a decision.
GLP-1 Drugs and Aging
Eric Topol (38:23):
Now, the drug class that's come on the scene, of course it was in incubating for decades for diabetes, but now obesity and so of the obesity related. But now we're seeing the GLP-1 drugs that are showing potential effects in Parkinson's and Alzheimer's and cardiovascular disease, and even in obesity related cancers. And I mean across the board. And you mentioned metabolic derangement as one of the things that accelerate aging. Do you think these class of drugs that has greatly passed our expectations already and it's being tested of course, with even more potent drugs or triple receptors and pills and whatnot, will that be a candidate as one of the anti-aging interventions in the future?
Steve Horvath (39:19):
Yeah, for sure. A couple of months ago, I participated in a conference and one of the speakers showed unpublished results from a study, and they looked good to me. I mean, they registered on epigenetic clocks. This is all unpublished, but it made perfect sense to me because I mentioned the clocks do relate to metabolic health. So I was quite pleased that they registered that intervention.
Eric Topol (39:56):
It's fascinating because we could all be taking GLP-1 drugs someday, not for obesity or not for sleep apnea, but for things that are more far reaching. I didn't know about that unpublished result. That's fascinating.
Steve Horvath (40:15):
Yeah, I have a joke, which is I wish I was chubby because I would be using these drugs, but I'm relatively slender, so I don't have any good reason to take them.
Eric Topol (40:28):
That says a lot. I don't know anybody who knows more about this process than you and is very candid and frank about it. So Steve, this has been terrific to have your insights, the body of work that you should be so proud of that extends over many years and many great years and more contributions to come undoubtedly. So thank you for joining us today, and we will follow this continued evolution of our ability, not just to track the aging process, but also to modulate. So thanks very much.
Steve Horvath (41:06):
Thank you. I really like your podcast Ground Truths, it’s very informative. So thank you for this.
********************************************************
We’re appreciative of your reading and subscribing to Ground Truths. All content of these newsletter/analyses and podcasts are free, with no advertisements.
Thanks to my producer Jessica Nguyen and Sinjun Balabanoff for audio and video support at Scripps Research.
Please share this post, especially if you found it interesting or informative.
If you do elect to be a paid subscriber, all proceeds are donated to Scripps Research and such support has already been extremely helpful for our summer internship programs and other educational activities. Comments and questions from paid subscribers are welcome.












Share this post