The Chief Scientific Advisor at Novo Nordisk, Lotte Bjerre Knudsen, was the key force who pushed hard to develop GLP-1 drugs for treating obesity and subsequently for Alzheimer’s. She was recently recognized by the 2024 Lasker Medical Research Award, and the 2024 AAAS Bhaumik Breakthrough of the Year Award. That recognition is richly deserved, since it is unclear if the GLP-1 drug path to obesity treatment, and all of the associated benefits, would have been seen at this time without her influence. That’s especially true given the mystery for why people with Type 2 diabetes (for which these drugs were used for many years) did not exhibit much in the way of weight loss. We discussed that and the future of these drugs, including their potential to prevent neurodegenerative diseases. And about dressing up in pink!
The Ground Truths podcasts are also available on Apple and Spotify.
Our entire conversation can also be seen by video at YouTube along with all of the Ground Truths podcasts. If you like the video format, please subscribe to this channel. Even if you prefer video, please take a look at the transcript with graphics and useful links to citations.
A Video Clip below on the barriers of a woman scientist to push Novo Nordisk to develop GLP-1 for obesity.
“I was always just been a nerdy little scientist who kind of found home here in this company for 35 years.”—Lotte Bjerre Knudsen, 60 Minutes
Transcript with Links to audio and external references
Eric Topol (00:06):
Well, hello, it's Eric Topol with Ground Truths, and I have with me a special guest. She's the Chief Science Officer of Novo Nordisk and it's Lotte Bjerre Knudsen, and we're delighted to have her. She's a recent recipient of the Lasker Award, which I think is considered like the pre-Nobel Award here in the United States. And I was involved with her in terms of researching who was the principal person who brought the GLP-1 drugs to the forefront for obesity, and it turned out to be Lotte. So welcome, Lotte.
Lotte Bjerre Knudsen (00:48):
Thank you very much. And also very, very happy to be here. I'm not the Chief Science Officer for Novo Nordisk, I'm the Chief Scientific Advisor of working for the Chief Science Officer of Novo Nordisk, but maybe too many people, not so different, right?
From Laundry Detergents to GLP-1 Drugs
Eric Topol (01:06):
Yes. Thank you, I actually meant to say advisor, but yes, I'm glad you cleared that up. I know from speaking to some of your colleagues, I actually spoke to Robin yesterday that you are looked to very highly, the most highly regarded person in science there, so not surprisingly. What I want to do is first talk about the glucagon-like peptide-1 (GLP-1) that got its legs back in, I guess 1984. So we're going way back. And what's also interesting is that you go way back at Novo Nordisk to 35 years in 1989. And so, there had been this work with this extraordinary hormone and neurotransmitter with a very short half-life that you knew about. But when you first started in Novo Nordisk, you weren't working on this. As I understand it, you're working on laundry detergent enzymes. How did you make this pivot from the laundry enzymes to getting into the GLP-1 world?
Lotte Bjerre Knudsen (02:16):
Yeah, thank you for that question. I'm from the technical University of Denmark, so I'm trained in biotechnology, and we're a small country, so not that many companies to work for. And I always had my mind set on, I wanted to work for Novo as it was called back then, and it just happened to be in the industrial enzyme part that I got my foot in first. And then I had a very interesting boss at the time. Unfortunately, he's not alive anymore, but he was both a medical doctor as well as a chemist. So he was actually put in charge of actually, let's see if we can do something new in diabetes. And then since he hired me and I had not been there that long, I simply tagged along as the youngest scientist on the team, and then suddenly I became a diabetes researcher. Around the same time, I think you remember that all of pharma was interested in obesity in the early 90s, everyone wanted to do diabetes as well as obesity, but they were separate teams and they all wanted to do small molecules, but it just happens to be so that the best idea we could find at that time was actually GLP-1, because we actually had clinical data relatively early that GLP-1 was a really good candidate as a treatment for diabetes because of the glucose sensitivity of the actions.
(03:43):
So you'd have efficient lowering of glucose through a dual mechanism with increasing insulin, lowering glucagon, and then it was safe because there wasn't this hypoglycemia you get from insulin. But then I had other colleagues who were working on obesity, and I was just kind of listening, right, what's going on there? And then also a colleague that I had, we had, I don’t know if you remember the old Hagedorn Research Institute, but Novo actually had kind of like an academic research institute that was affiliated with us. And there was this group that were working on this glucagon tumor model that produced high levels of glucagon, GLP-1 and PYY. And these rats, they starved themselves to death. And I knew about that from 1994. So that actually inspired my thinking. So when Stephen Bloom's paper came out in January of 1996, and he was the first one to call GLP-1 a neurotransmitter, I think, but I was already way into actually screening these kind of molecules that later then became liraglutide.
No One Else Thought About This [Obesity]
(04:54):
And then I thought, why on earth should we not actually do both things at the same time? If we have an idea that can both work in diabetes in a much safer way than in insulin, and then also at the same time work in obesity. But the reality is that no one else thought about this, or if they thought about it, they didn't really think that it would a good idea. But I think I had the luxury of being in a biotech company, so everyone was working with peptides and proteins. So I don't think I got the same challenge that the other people in the other pharma’s got when they all wanted small molecules.
Eric Topol (05:36):
Well, also just to set the foundation here, which you alluded to, there had been so many attempts to come up with a drug that would work, not just of course in diabetes where there are many classes of drugs, but moreover, to treat the condition of obesity. Actually, I was involved with one of them, Rimonabant and did the large trial, which as you know, led to having to stop the drug, discontinue it because it was associated with suicidal ideation and actual some suicide. So there had been such a long history of checkered inability to come up with a drug. But what was striking is the challenge, and this is one of the first important questions about, when you had the extended half-life of the first GLP-1 drug, that instead of having to take multiple times a day, you could actually, with liraglutide get to a point where you were starting to get to an extended half-life. This is now going back to 1997 with approval in 2010, still 14 years ago. But when you came up with this drug, because this was certainly one of your great contributions, this drug was just a step along the way in this kind of iterative process, wouldn't you say? It wasn't the long half-life and the potency that eventually got us to where we are today. Is that true?
Lotte Bjerre Knudsen (07:15):
Yeah, it was a stepwise process. And what's super interesting about this class of medicines is that they're actually so different. If you talk about a class of medicine where small molecules, they can be different, but they're usually more alike than they're different. And when it comes to this class with these medium-sized peptides, people tried a whole bunch of different things. So they're actually really, really different. Some are simple peptides. So the idea that I came up with was to use this fatty acid isolation principle, and that's then a subclass in the class. And then the first, once weekly, for example, was an antibody-based molecule liraglutide. So they're much, much, much larger molecule compared to the small peptides. So they're very different. And neither the simple peptides nor the really big antibody derived molecules, they don't give a lot of weight loss. So we actually get more weight loss with these kinds of molecules, which is also why you can now see that it has actually kind of inspired a whole industry to kind of try and go and make similar kinds of molecules.
Eric Topol (08:27):
Well, inspired a whole industry is an understatement. It’s become the most extraordinary class of drugs, I think in medical history, having been a student of various, I mean obviously statins have been a major contribution, but this seems to have transcended that already. We're going to talk about more about where things are headed, but this fatty acid acetylation was a major step forward in extending the half-life of the drug, whereby today you can give semaglutide once a week. And this, I think, of course, there are many ways that you might've been able to extend the half-life, but you were starting with a hormone, a natural hormone neurotransmitter that had such an exquisitely short half-life of basically second or minutes rather than that you could give for a week. So I know there were many different ways you could have protected or extended the half-life one way or another, but this seemed to be a breakthrough of many along the chain of breakthroughs. But the question I have is when you were giving this to the diabetics, which was the precedent, that was really what these drugs were first intended, they didn't lose that much weight, and they never, still today when it's looked at for obese non-diabetics versus diabetics, there's a gap in weight loss. Why is that at the exact same dose, with the exact same peptide that the weight loss differs for people with type 2 diabetes as compared to those who have pure obesity?
The Mystery of Why People With Type 2 Diabetes Don’t Lose Weight Like Those With Obesity
Lotte Bjerre Knudsen (10:09):
Yeah, I can't give you a molecular answer to that, right. But I think the notion, I think it's the same for example with metformin, even though it gives less weight loss because that has also been tried in both people with diabetes and people without diabetes. So I think it's just for somehow people with diabetes are more resistant to weight loss. I think it's a really good question that I'm hoping maybe we could get through, for example, with proteomics and actually comparing people with diabetes and people without diabetes and looking at people who have the similar kind of weight loss. That could be really interesting. But I really don't have a good molecular answer for you, but it's just a really, really strong fact. But it also leads me to wanting to say it’s interesting, because if that had been our motivation to actually say, oh, there's weight loss in diabetes, let's pursue it in people with obesity, I don't think we would've done that because the weight loss in people with diabetes wasn't that impressive. So it was very important for our chain of thought and decision early on that we actually knew that GLP-1 had these separate effects and that they could work in the brain and have a separate effect on well-known pathways in the brain. And that was more our motivation to actually continue to invest in obesity.
Eric Topol (11:42):
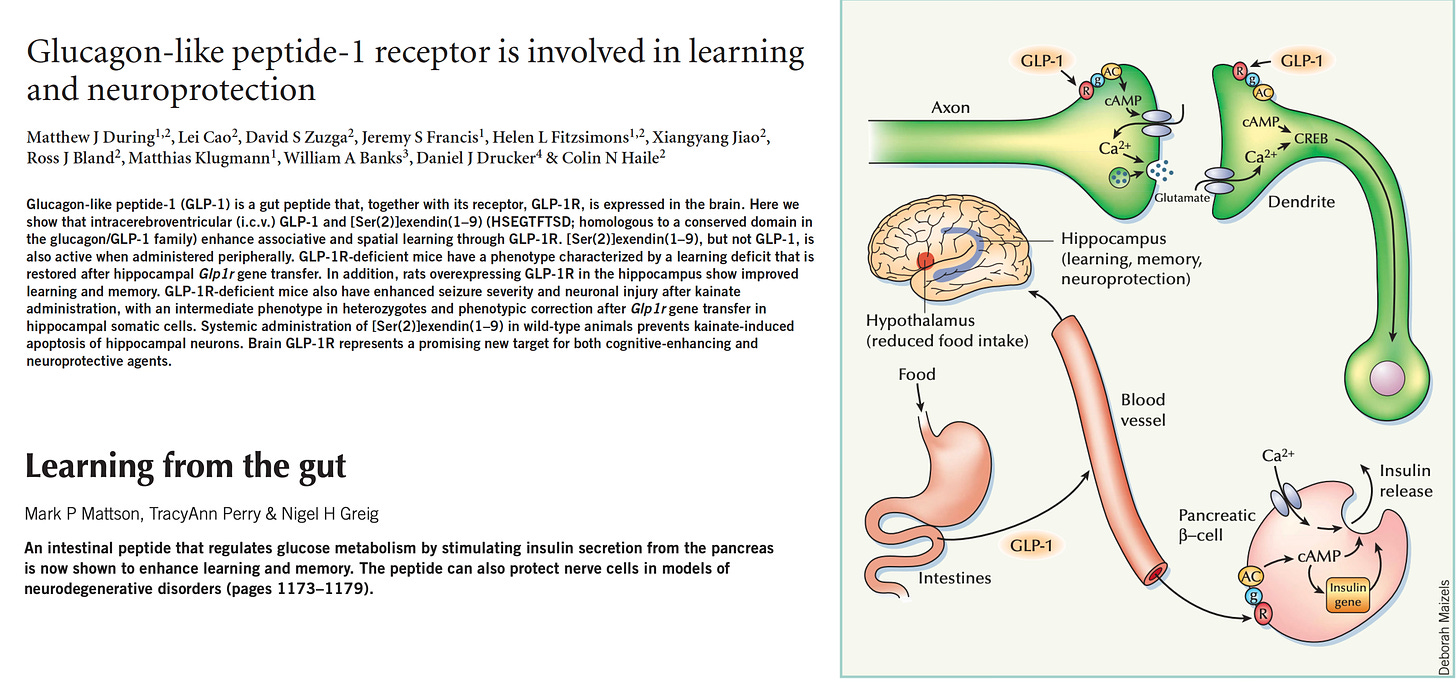
Yeah, no, I think this is when we did the research on the committee for the American Association for Advancement of Science (AAAS) award, the Mani L. Bhaumik Award, that you were recognized for the breakthrough of the year, this year. We tried to scour all the work and we actually had to hit Danish translations and all sorts of other papers they reviewed. And we learned through that process working on this committee that you were the one to be the champion of pushing this towards obesity, and it would've easily been missed because as we've been discussing, the weight loss in people with diabetes was small, but you push for it. And this was an extraordinarily important push because what it has resulted in, of course, has been spectacular. And obviously as we're going to get into much more than just obesity and obesity related conditions. But before we get to those other conditions, and as you've been known in the medical community as “the mother of GLP-1”, you were dubbed that term. The GLP-1 receptor is expressed in many parts of the body. Maybe you could just tell us about the distribution because this, I think is tied into these central nervous system effects that are not just related to the gut hormone type of axis.
GLP-1 Receptors and the Brain
Lotte Bjerre Knudsen (13:17):
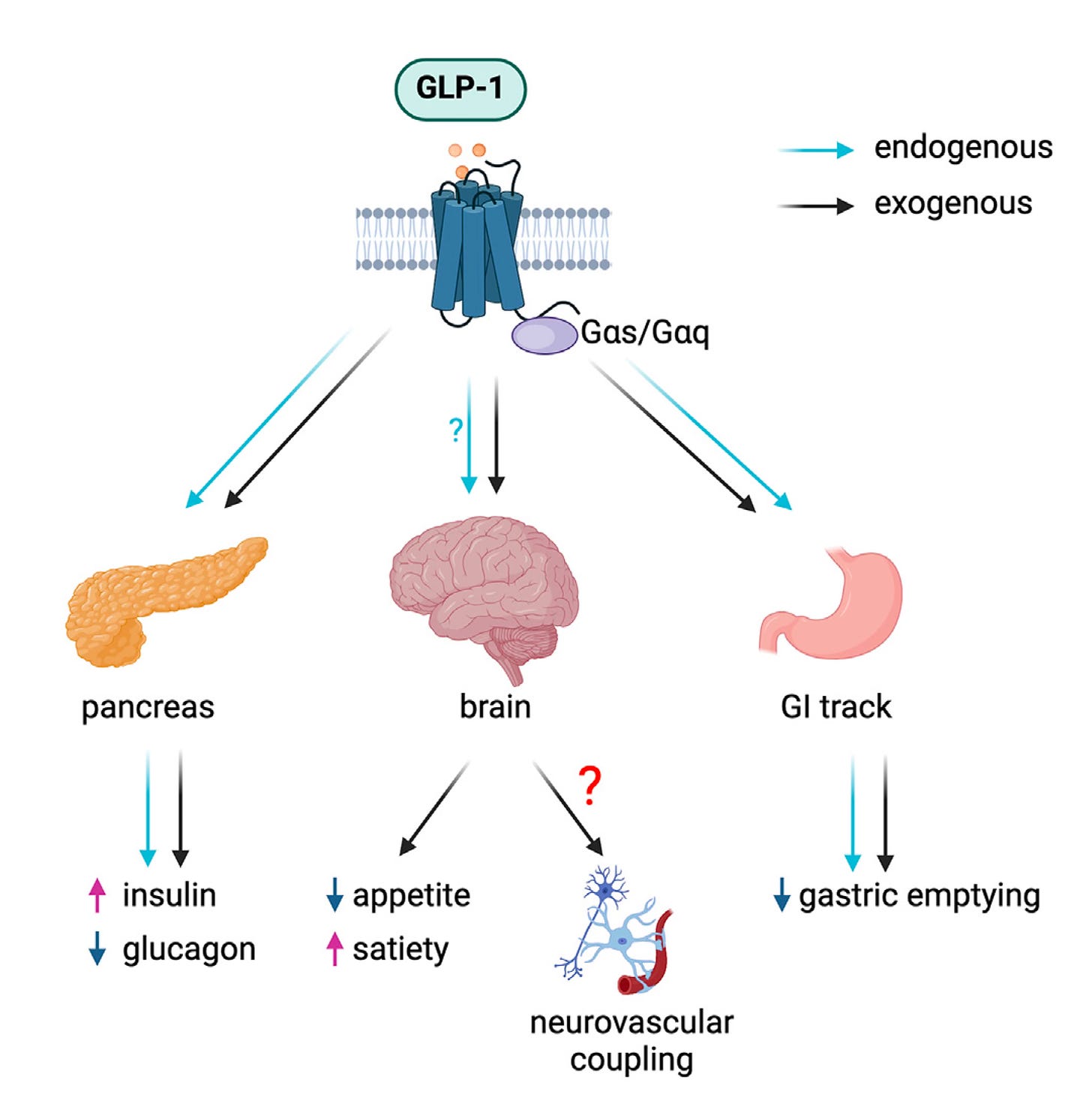
So I spent a lot of time on that together with my amazing colleague, Charles Pyke, who's an histology expert because it turned out to be so very important. In general, when you're trying to make new medicines, understanding the mechanism, sometimes people say, yeah, who cares? But actually, it should matter, I think because where it becomes really important can be an understanding what they do not do. We've had to do a lot of proving the negatives for GLP-1. We went through these issues with thyroid cancer, pancreatitis, pancreas cancer. In all of that work, it was actually really important that we could show where the GLP-1 receptor was not expressed. So in the pancreas, we know that it's primarily on the insulin producing cells, and then we also have them in the intestine where they're probably involved in regulating inflammation and really creating a much healthier gut.
(14:15):
And then we have a lot of receptors in the brain. They're typically expressed on neurons, but they're also on astrocytes, they’re also on smooth muscle cells. We have them on the heart and the sinus node. That's why there's a small increase in heart rate. We have them in the kidney, on again some smooth muscle cells that are renin positive. So there we can start thinking blood pressure and other things. So it turns out that you can go around the body and there are all of these specific GLP-1 receptor population, that you can see how they tie into the pharmacology. But obviously in physiology, they're not as important as they have turned out to be in pharmacology when we suddenly come with 24 hours a day exposure for a day or a week or for as long as the administration interval is. So, but specifically for obesity, I think it's in the vein, it's hard to, you should always be careful.
(15:18):
That's something I've learned to never say never. Of course, there could be a contribution from the peripheral nervous system as well to the effects in obesity. But I do think there are so many important and well described neuronal populations that have the GLP-1 receptor and which are accessible from the periphery. So just to mention, maybe one of the most, well-known is a POMC/CART neuron in the hypothalamus. They have the GLP-1 receptor, they're activated, but there also is an inhibitory tone on the AgRP and NPY neurons, and it fits very well with that. We know that people report that they feel more sated, they feel less hungry. But then there are also effects in the hindbrain and in some of the reward centers also have GLP-1 receptors. And we know that also now, we have really good actually clinical studies that show that there is a change in food choice and people can control their food intake better. So I think that fits very well with effects on the reward system. So it's a whole myriad, or maybe you could say that GLP-1 orchestrates a number of different neuronal populations to have these overall effects that reduce energy intake.
Eric Topol (16:42):
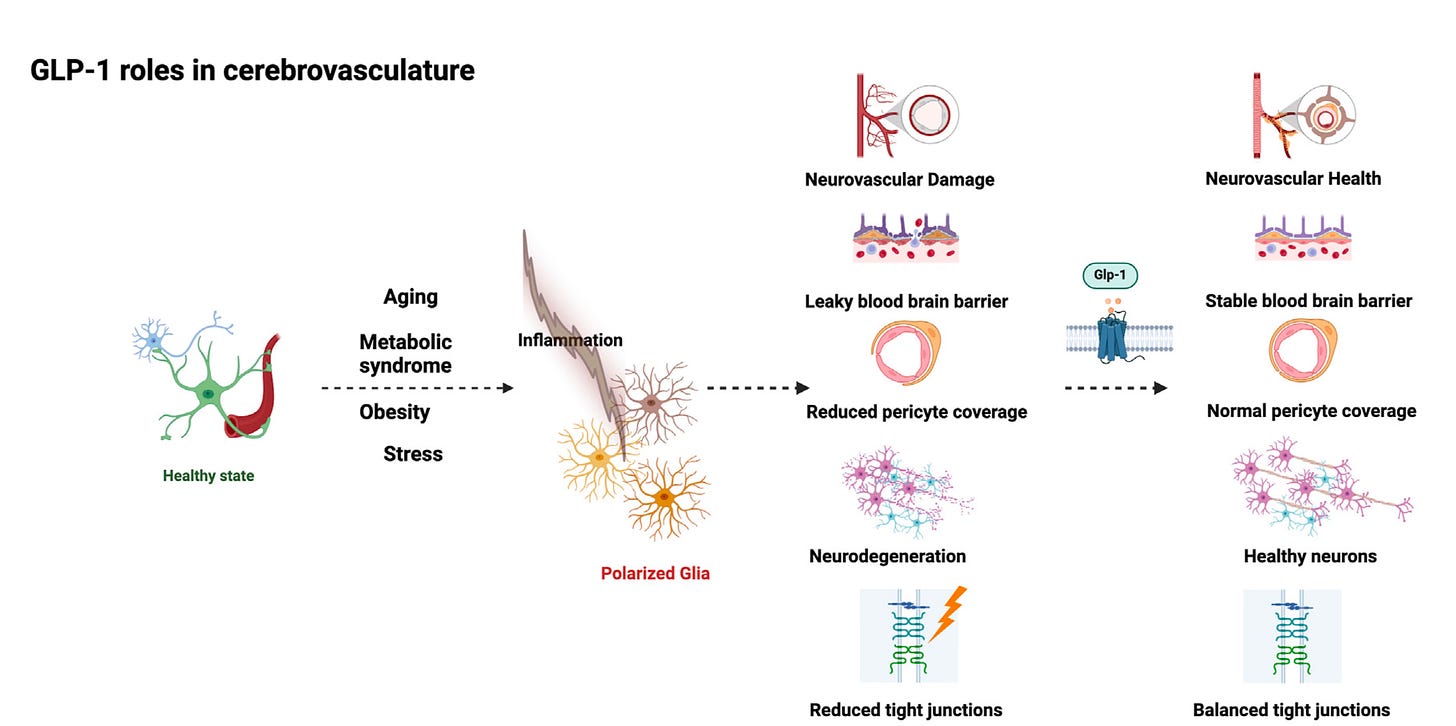
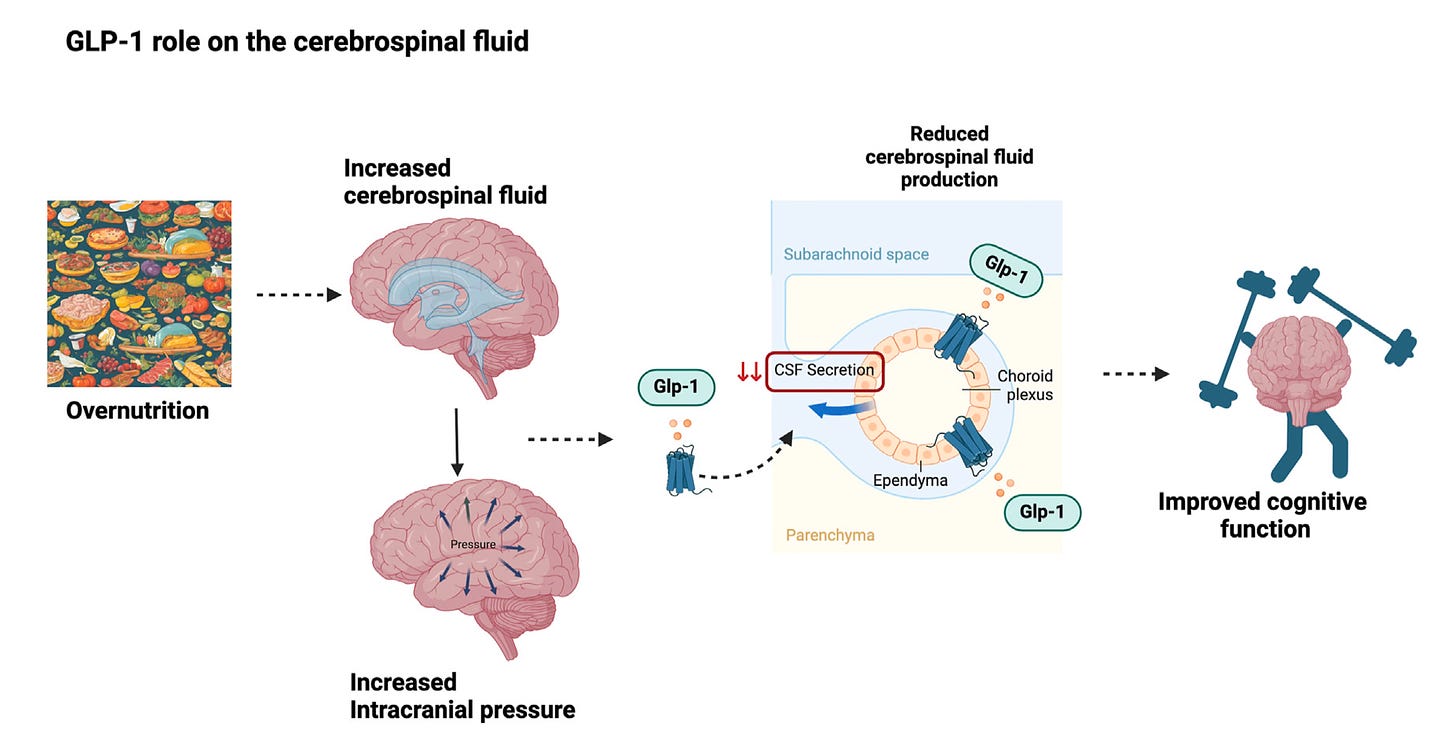
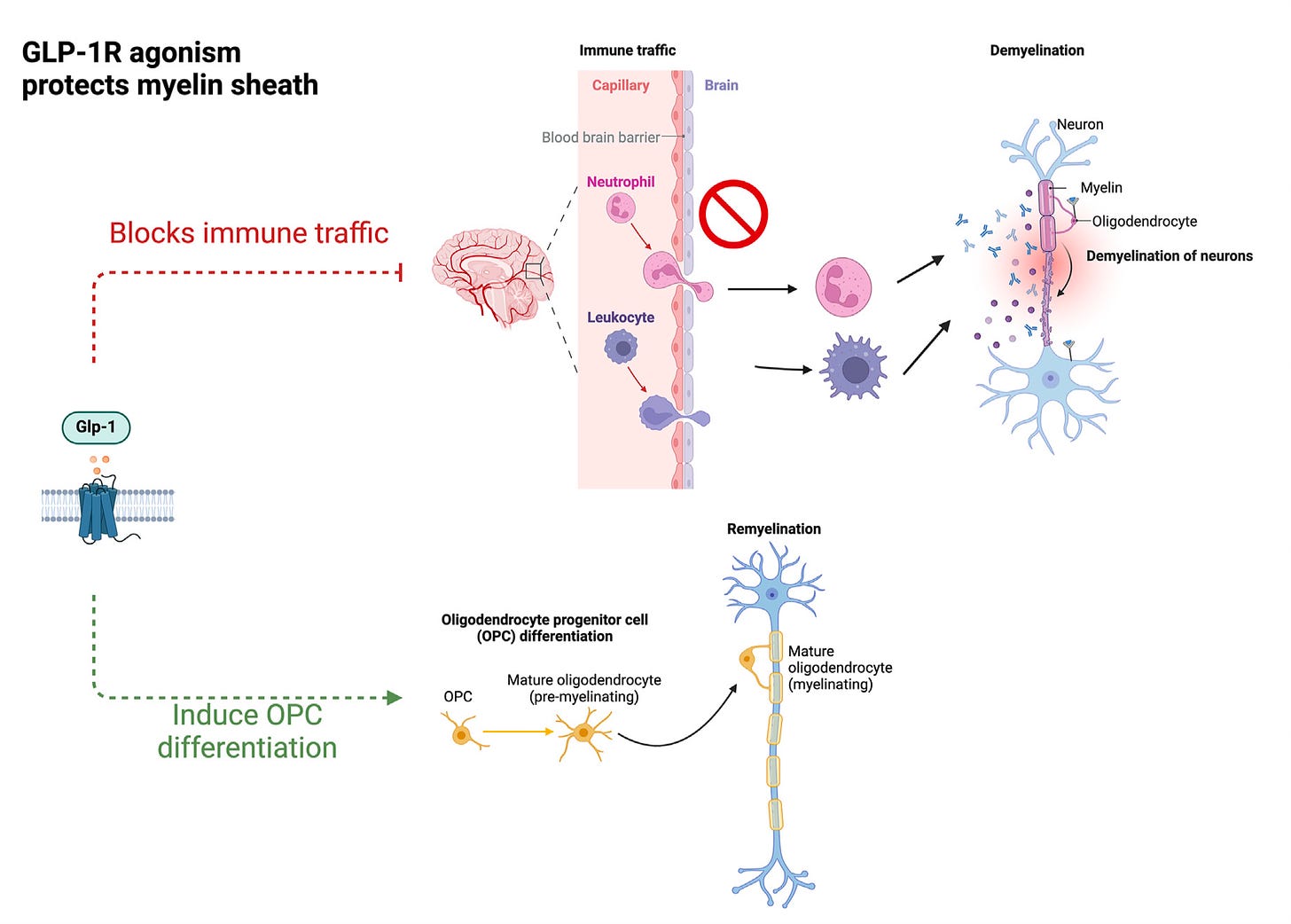
Yeah, it's pretty striking. It's almost like we're all walking around with GLP-1 deficiency, that if we had this present at higher levels around the clock, and of course eventually we'll see things that are well beyond obesity, how well this has an impact. Now, there was an extraordinary review in Cell Metabolism on the brain and GLP-1, and not just the brain, but the essential nervous system, the neurovascular, it's called the “GLP-1 programs and neurovascular landscape.”
(17:20):
And in this review, it got into the brain effects that were well beyond, I think what are generally appreciated. Not only the protection of the integrity of the blood-brain barrier, this whole neuroglial vascular unit, the myelin sheath protection, reducing inflammation within the brain, improving the glymphatic flow, which is of course critical for clearing waste and promoting cerebral vascular remodeling and more, so the brain effects here is what it seems to be. You mentioned the reward circuit, of course, but the brain effects here seem to be diverse, quite a bit of breath and extraordinary. And as we've seen in the clinic now with the work that's been done, we're seeing things about addiction, even gambling, alcohol, drugs, I mean neuropsychiatric impact, it's pretty profound. Maybe you could comment about that.
On to Alzheimer’s and Parkinson’s Diseases
Lotte Bjerre Knudsen (18:23):
Yeah. I haven't read that paper yet, but I just saw it earlier. And I have been following this for about actually more than 10 years because when I was kind of over the big work of actually getting the approval for diabetes and obesity. I thought I had a little bit of capacity to actually look at Alzheimer's and Parkinson's disease because I just thought there's such an insane unmet need and what if GLP-1 could actually make a difference? And the first big paper that talked about this was actually in Nature Medicine in 2003, and it was originally, I think I should credit Nigel Greig. Greig, he's from NIH or from NIA, I can't remember, right. But he was actually the first one, I think to say if GLP-1 has all of these important effects in the pancreas and to protect cells, and there are all these GLP-1 receptors in the brain, maybe it also protects neurons.
(19:25):
So that was the first hypothesis. And the paper on Nature Medicine in 2003 describes how the GLP-1 receptor in the hippocampus is involved in cognition. And then we did a couple of studies in different animal models, and I was, to be honest, really confused. But then there was a new paper in Nature Medicine in 2018 that started to focus in on neuroinflammation. And by that time, I knew much more about inflammation and knew GLP-1 actually lower CRP by about 50% in the different trials. So I was really tuned into the potential importance of that in cardiovascular and kidney disease. But I was like, oh, what if that's also something that is important in the brain? Then it made more sense to me to try and build some evidence for that. So that was how we actually started looking at a hypothesis for Alzheimer's and Parkinson’s.
(20:21):
And we now have a really large phase three study ongoing, but of course, it's a hypothesis, right? And no one has yet, I think, proven that GLP-1 has really important effects on these indications, but we are testing it in 4,000 people with Alzheimer's disease. So our hypothesis is around neuroinflammation, but defined in a way where you could say it's both peripheral inflammation and the effect it has on the vasculature, it's the effect on the blood-brain barrier. It's the astrocytes and the microglia, and there are probably also some T cells that have the GLP-1 receptor that could be important. And then couple that up also with some of the new information from neurons, because there are two papers to think in the last year that has highlighted neurons either in the hindbrain or a little bit further on. Both of them are probably hindbrain populations that actually seem to be really important in regulating both peripheral as well as central information.
(21:27):
So what if neurons are actually also an overlooked mechanism here, and both of these neuronal populations have the GLP-1 receptor and are accessible from the periphery, even though the child super paper in Nature doesn't mention that, but they do have the GLP-1 receptor. So there are all these different mechanisms that GLP-1 can have an impact on the broad definition maybe of neuroinflammation. And maybe the way one should start thinking about it is to say it's not an anti-inflammatory agent, but maybe it induces homeostasis in these systems. I think that could maybe be a good way to think about it, because I think saying that GLP-1 is anti-inflammatory, I think that that's wrong because that's more for agents that have a really strong effect on one particular inflammatory pathway.
Eric Topol (22:22):
That's a very important point you're making because I think we conceive of these drugs as anti-inflammatory agents from these more diverse actions that we've just been reviewing. But I like this restoring homeostasis. It's an interesting way to put it. This brings us, you mentioned about the Parkinson's, and when I reviewed the three randomized Parkinson's trials, they're all small, but it appears to be the first disease modifying drug ever in Parkinson's. Of course, these were done with different drugs that were older drugs. We haven't seen the ones that yet to be with semaglutide or other agents. And I wondered if you pushed, just like you did for obesity within Novo Nordisk, you pushed to go into obesity. Did you also force to push for Alzheimer's?
Lotte Bjerre Knudsen (23:19):
Yes. So that is also me who had to argue for that. I'm happy to do these things. I was born brave. I am happy to do these things.
Eric Topol (23:31):
That's wonderful. Without you, we would be way behind, and it took decades to get to this point. But look where we are now, especially with all the rigorous trials, the large clinical trials. You're into one right now of some 20,000 participants to see whether not just people with prior heart disease, but people without known heart disease to see whether or not this will have an effect. And there's so much data now, of course, already a completed trial with reduction of heart attacks and strokes. But now to extend this to people who are not such high risk, but these large trials, we keep learning more. Like for example, the reduction of inflammatory markers is occurring even before the weight loss that starts to manifest. So we learned a lot from the trials that are just even beyond some of the major primary outcomes. Would you agree about that?
Lotte Bjerre Knudsen (24:34):
So I'm not sure we can say that it comes before the weight loss because the energy intake reduction happens instantly. The glycemic response happens instantly. And all of these improvements will of course also have an effect to dampen inflammation. We do not have data that supports that it comes before because we haven't sampled that much in the beginning.
Eric Topol (25:04):
Okay.
Lotte Bjerre Knudsen (25:05):
I wouldn't be able to say that, and I don't think there are any, well, it's hard to keep up that the entire literature on GLP-1 these days, but I don't think anyone has actually shown that there is a separation because it's super hard to separate when things are occurring at the same time.
Eric Topol (25:24):
Yeah, I'm just citing the heart disease trial where in the New England Journal that point was made. But I think your point also that there was already a change in energy intake immediately is apropos for sure. Now, when we get into this new paper of yours, the proteomics, can you tell us about that because that's really exciting. We're in a high throughput proteomics era right now that we can analyze thousands of plasma proteins in any given individual. What are you learning about proteomics with the GLP-1 drug?
The GLP-1 Drug Impact on Proteomics
Lotte Bjerre Knudsen (26:07):
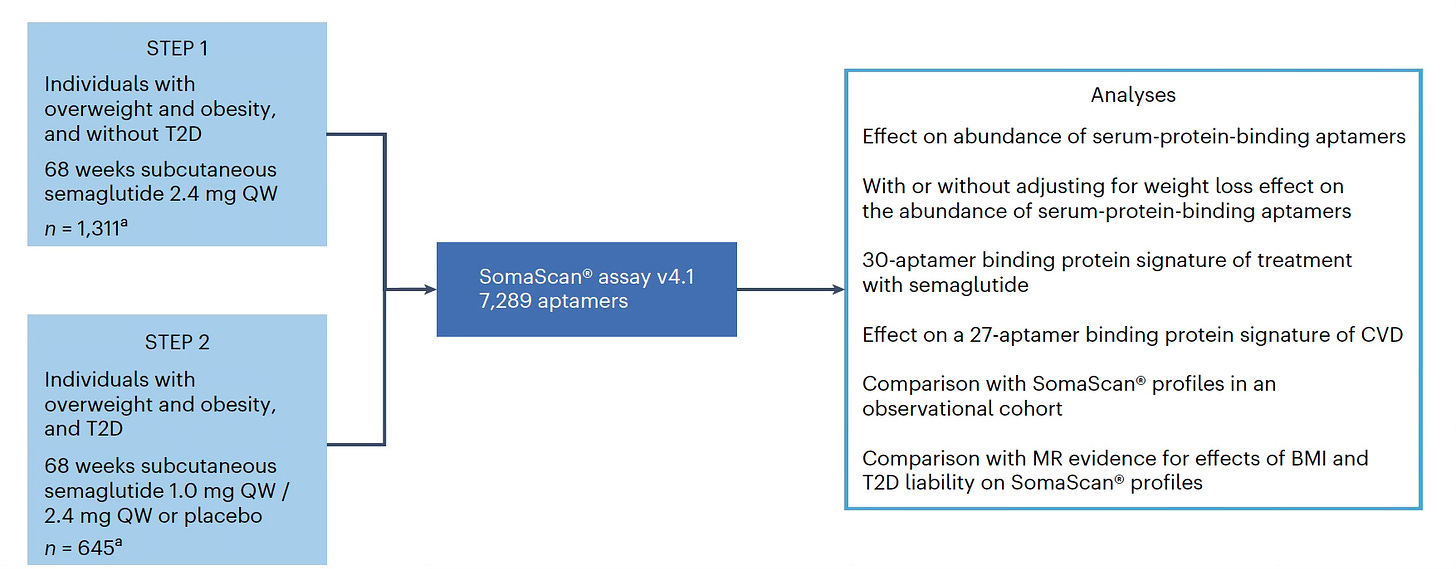
Yeah, yeah. So I'm also the super excited about omics, right? Because I have worked in a wonderful organization of people who can do these large scale clinical trials, and we used to not collect a lot of samples for future use, but we've done that for some years now. So now we have this amazing collection of samples we can learn from and actually both inform the patients and the physicians, but also inform future research. So we have been doing that in our semaglutide trials, and we've just published the proteomics data from the step one and step two trials. So the phase 3a trials that supported the approval of semaglutide for the treatment of obesity. So one of them in people with obesity and one in people with obesity and diabetes, and those data are now published in Nature Medicine. [3 January 2025]. And we were learning a lot of things because you can compare the proteome effects to what has been done in the decode cohort.
(27:11):
So they have all these disease signature. So that's one thing that you can for sure see, and you can see a lot of things there with hints towards addiction. And then also you can take more predefined signatures also to look into what actually might be driving the cardiovascular risk. So I think there are so many things that you can learn from this, and of course it can also inform when you look at what's actually mediating the effect and probably something around inflammation is important. We have already also shown a more standard mediation analysis that shows that actually the most explainable factor for the effect on MACE [major adverse cardiovascular events] in the select trial is inflammation. It doesn't explain everything, but it actually looks like it's more important than BMI and weight loss. So that's really interesting how much we can learn from there. We're making the data are available at the summary statistic level so people can go and play with them ourselves.
(28:23):
And I think as we have more different kinds of medicines available in obesity, it's also a way to kind of compare how these different medicines work. And as we get more and more better at maybe also characterizing people with obesity, because I think that's a great thing that's going to happen now is there's going to be more funding for obesity research. Because I think that's what the attention that we are seeing right now is also giving. Then we can better start to understand. We always, we've been saying that people probably have different kinds of obesity, but we don't really know. So now we can actually start to understand that much better and maybe also understand how these different classes of medicines will work if we have the proteome data from different trials.
Eric Topol (29:10):
No, I'm absolutely fascinated about the proteomics. I call it a quiet revolution because many people don't know about it. [My recent post on this topic here.]
(29:18):
The ability to assess thousands of proteins in each individual, and it's giving us new insights about cause and effect as you alluded to, the relationship with as you said, MACE (major adverse cardiovascular events) and the actions of this drug class. I mean, there's just so much we can learn here from the proteomics. Another thing that's fascinating about the GLP-1 is its effect on epigenetic clocks. And recently at one of the meetings it was presented, this is Steven Horvath that we had on Ground Truths not long ago. He talked about at this talk that for the first time to see that you could basically slow the epigenetic clock with a GLP-1. Is there any further information about that?
Lotte Bjerre Knudsen (30:16):
Yeah, no. We've never had enough of a sample size to actually be able to look at it, so unfortunately, no. But there is something else, right, because there is this group at the Stanford, Tony Wyss-Coray or something.
Eric Topol (30:33):
Yes, Tony Wyss-Coray.
Lotte Bjerre Knudsen (30:35):
Now he published a paper, is it two years ago? Where he did it using proteomics. He defined an anti-aging signature for various different organs.
Lotte Bjerre Knudsen (30:46):
We are in the process of trying to see if we could take those signatures and apply them on to our data.
Eric Topol (30:55):
Well, what's interesting is we're pretty close friends, and he, not only that paper you mentioned on organ clocks, which is a phenomenal contribution, but he has a paper coming out soon in Nature Medicine, the preprint is up, and what he showed was that the brain and the immune system was the main organ clocks that were associated with longevity. And so, it takes another step further and it's looking at 11,000 plasma proteins. So it's really interesting how this field is evolving because the omics, as you put it, whether it's proteomics, and now we're learning also about the epigenome and what brings us to the potential that this class of drugs would have an impact on health span in all people, not just those who are obese. Would you project that's going to be possible in the years ahead?
Lotte Bjerre Knudsen (32:02):
I don't know about health span, but because certainly there's been so many studies with metformin and there's been a lot of wonderful data showing an effect on the epigenetic clocks, but not really an effect on lifespan because that metformin is so widely used. If that was the case, it would be easy to dig those data out of different registries. But certainly a healthier aging is the most obvious one because when you have one class of medicine that actually has so many different effects. Right now we are looking at them at a one by one case, but we really should be looking at them so you are getting the benefits on the heart and the vasculature on the brain and the kidneys and the diabetes and the knees. You're getting all of that at the same time, and that certainly should lead to much, much healthier lives. And then of course, we just need to get people to eat healthier. Also, maybe we should talk a little bit about the food industry. I heard you did that in some of your podcast, right?
Eric Topol (33:17):
Yes. That is the big food, if you will. It's a big problem, a very big problem, and the ultra-processed foods. And so, lifestyle is not good and trying to compensate for that with a drug intervention strategy is like chasing your tail. So you're absolutely right about that. I mean, I guess what I'm getting into here is that whereas today we keep seeing the effects, whether it's the liver, the kidney, the heart, obesity, and people with diabetes. But for example, in the Alzheimer's trial, do you have to be obese to be enrolled in the Alzheimer's trial, or is it just people who are at risk for developingAlzheimer's?
Lotte Bjerre Knudsen (34:01):
Yeah, no, you do not have to be obese. It's a standard Alzheimer's trial.
GLP-1 Pills
Eric Topol (34:07):
So this will be one of the really important trials to get a readout in people who are not having an obesity background. Now, the future, of course, gets us to oral GLP-1 drugs, which obviously you have there at Novo Nordisk. And it seems to me once that happens, if it can simulate the effects we see with the injectables, that would be another big step forward. What do you think about that?
Lotte Bjerre Knudsen (34:39):
Yeah. Isn't it interesting, what we've learned is that people actually don't mind the injections, right? Also, because I think it's simple, once a week injection and the needles are so small, obviously there are people who really have needle phobia, but take those aside, it's relatively few. I would argue if you close your eyes and somebody else used this needle on you, you would not be able to feel where it was inserted, right? They're so small. So it becomes maybe a personal preference. Would you like to have once a day or maybe twice a day tablets, or are you fine with once a week injection? And I think there probably will be quite a few once they've tried it. And now so many have tried it and they actually, maybe it gives us a simple lifestyle. You don't have to do it every day, right? You can just have a weekly reminder.
Eric Topol (35:46):
Yeah, no, I think that's really interesting what you're bringing up. I never thought we would evolve to a point where injectables were becoming some common, and I even have some physician colleagues that are taking three different injectable drugs.
Lotte Bjerre Knudsen (36:00):
That's also just mentioned Richard DiMarchi, who I shared the Breakthrough Prize with, and also Svetlana Mojsov, who I was one of the other two recipients for the Lasker prize because they both been at Rockefeller, and they both have worked a lot with peptides, and they both say the same thing. They were told so many times, this is not medicines, these kinds of molecules just they're not medicines. Forget about it. It turns out people were wrong. And peptides can be medicines, and they can even be produced also in a sustainable manner with fermentation, which is not a bad way of producing medicines. And people actually don't mind. Maybe some people actually even like it because it's once a week and then it's done.
Confronting Barriers
Eric Topol (36:58):
Yeah, no, that's a very important point. And the quest for the oral, which have more issues with bioavailability versus the peptides that are having such pronounced impact is really interesting to ponder. Well, before we wrap up, it's very clear the impact you've had has been profound, not just obviously at Novo Nordisk, but for the world of advancing health and medicine. And you've mentioned some of the key other people who have made seminal contributions, but I think you stand out because when we went deep into who took this field forward into obesity and who might also wind up being credited for Alzheimer's, it was you. And as a woman in science, especially in an era that you've been at Novo now for three and a half decades, there weren't many women in science leaders. And for one to be, as you said, you're brave for the good old boys to listen to the woman in science. Tell us about that challenge. Was this ever an issue in your career? Because obviously we want to have this whole landscape change. It is in the midst of change, but it's certainly still a ways to go. So maybe you can give us insight about that.
Lotte Bjerre Knudsen (38:27):
Yeah. Well, it for sure was a thing. It was a very male dominated world, and in a way, it might have prevented other people from doing it. But then, as I said, I was born brave for some reason. I'm not really sure why. It actually motivated me to kind of like, yeah, I'm going to show them. I'm going to show them. So it never really got to me that people, not everyone was nice to say. There was the first 10 years of my career, I think they were quite lonely, but then I was really inspired. I was so happy to be allowed to work on this. I thought it was super fun. And I did find people who wanted to play with me. And I also have to say that the CSO back then, Mads Krogsgaard Thomsen, he always supported me. So maybe I didn't get everything I wanted, but I always got what I needed in order to progress.
(39:29):
So on the women's side, and I think that yes, and there's still a change to be made, and I'm actually a little bit on behalf of my generation, maybe not too proud of the change we made because we didn't do a lot of change. It was all the women coming from the arts and the culture. They were the ones who actually make the big change here like 5 or 10 years ago. So I've also started to be more open about sharing my journey and advocating for women in science. So that's why I show up in pink to some of these award sessions just to be a little bit different and to maybe also just show that you don't have to be a certain type in order to fit into a certain job. But there is still a change to be made where people should be better at listening to what a person say and what ideas they say.
(40:28):
And they should be mindful about not always labeling women as passionate. When people call me passionate, I say like, no, thank you. I'm actually not too happy about the mother of either, because men always are being told. They're being told that they're brave and ambitious and courageous and strategic, whereas we we’re, oh, you're so passionate. No, thank you. I'm also brave and strategic and ambitious and all of that. So we simply put different vocabulary on. I don't think people don't do it on purpose. I think we need to be better at actually giving people at work the same kind of vocabulary for their contributions. And I think that would mean that we get listened to in the same way. And that would be important. And then I also have to say that science, whether it comes from men or women, doesn't really matter.
(41:32):
Successful science is always the work of many. And I hope that some of you will actually listen to my last speech because that's what I speak about, how it's always the work of the many. And also, how if you want to do something novel, then you actually have to do it at a time when no one else is doing it, and you should believe in your ideas. So believe in it, listen to the critique, but believe in it, and then come back with new arguments or give up if you can't come up with any new arguments, right?
Eric Topol (42:05):
Well, we'll definitely put a link to the Lasker Awards speech that you gave. And I just want to say that the parallels here, for example, with Kati Karikó , my friend who had the Nobel Award for mRNA, she spent three decades trying to get people to listen to her and never got a grant from the NIH or other places [our conversation here]. And it was a really tough battle. And as you already touched on Svetlana Mojsov, who did some of the seminal work at Rockefeller to isolate the portion of GLP-1, that really was the key part peptide, and it was overlooked for years. And so, it's a tough fight, but you're paving the way here. And I think the contributions you've made are just so extraordinary. And I hope that over the years we will continue to see this momentum because people like what you've done, deserve this extraordinary recognition. I'm glad to see. And the Lasker Award is really capping off some of that great recognition that is so well deserved. We’ve covered a lot of ground today, and I want to make sure if I missed anything that you wanted to get into before we wrap up.
Lotte Bjerre Knudsen (43:30):
I think we've been around all the exciting biology of GLP-1, both in diabetes, obesity, cardiovascular, kidney, potential in Alzheimer's and addiction. We'll see, we need the clinical data and we've put out a message to inspire people to do new science. There's still a lot of unmet need out there. There's a lot of diseases that don't have good treatments. Even in the diseases we've talked about there’s a lot of money for diabetes. There are no disease modifying therapies for diabetes. It's not really changing the course of the disease. So there's a lot of things that needs great scientists.
Eric Topol (44:17):
And I guess just in finishing the discovery of this class of drugs and what it's led to, tells us something about that, there's so much more to learn that is, this has taken on perhaps the greatest obstacle in medicine, which was could you safely treat obesity and have a marked effect. Which decades, many decades were devoted to that and gotten nowhere. It's like a breakthrough in another way is that here you have an ability to triumph over such a frustrating target, just like we've seen with Alzheimer's, of course, which may actually intersect with Alzheimer's, with a graveyard of failed drugs. And the ones that it were approved so far in certain countries, like the US are so questionable as to the safety and efficacy. But it gives us an inspiration about what is natural that can be built on the basic science that can lead to with people like you who push within the right direction, give the right nudges and get the support you need, who knows what else is out there that we're going to be discovering in the years ahead. It's a broad type of lesson for us.
Lotte Bjerre Knudsen (45:38):
Yeah, there is another hormone that's also in phase three clinical development, right? The amylin hormone. We've had pramlintide on the market for years, but we have this long-acting version that is in phase three clinical development. That could be the same kind of story because there's also additional biology on that one.
Eric Topol (45:58):
Yeah, this is what grabs me Lotte, because these gut hormone, we've known about them, and there's several more out there, of course. And look what they're having. They're not just gut hormones, like you said, they're neurotransmitters and they're body-wide receptors waiting to be activated, so it's wild. It's just wild. And I'm so glad to have had this conversation with you. Now, congratulations on all that you've done, and I know the Nature Medicine paper that just came out is going to be just one of many more to come in your career. So what a joy to have the chance to visit with you, and we'll be following the work that you and your colleagues are doing with great interest.
Lotte Bjerre Knudsen (46:45):
And thank you very much, and thank you for your wonderful podcast. They’re really great to listen to on the go. Very easy listening.
*****************************************
Please complete the quick poll question above.
Thank you for reading, listening and subscribing to Ground Truths.
If you found this podcast informative please share it!
All content on Ground Truths—its newsletters, analyses, and podcasts, are free, open-access.
Paid subscriptions are voluntary and of course appreciated. All proceeds from them go to support Scripps Research. Many thanks to those who have contributed—they have greatly helped fund our summer internship programs for the past two years. I welcome all comments from paid subscribers and will do my best to respond to each of them and any questions.
Thanks to my producer Jessica Nguyen and to Sinjun Balabanoff for audio and video support at Scripps Research.
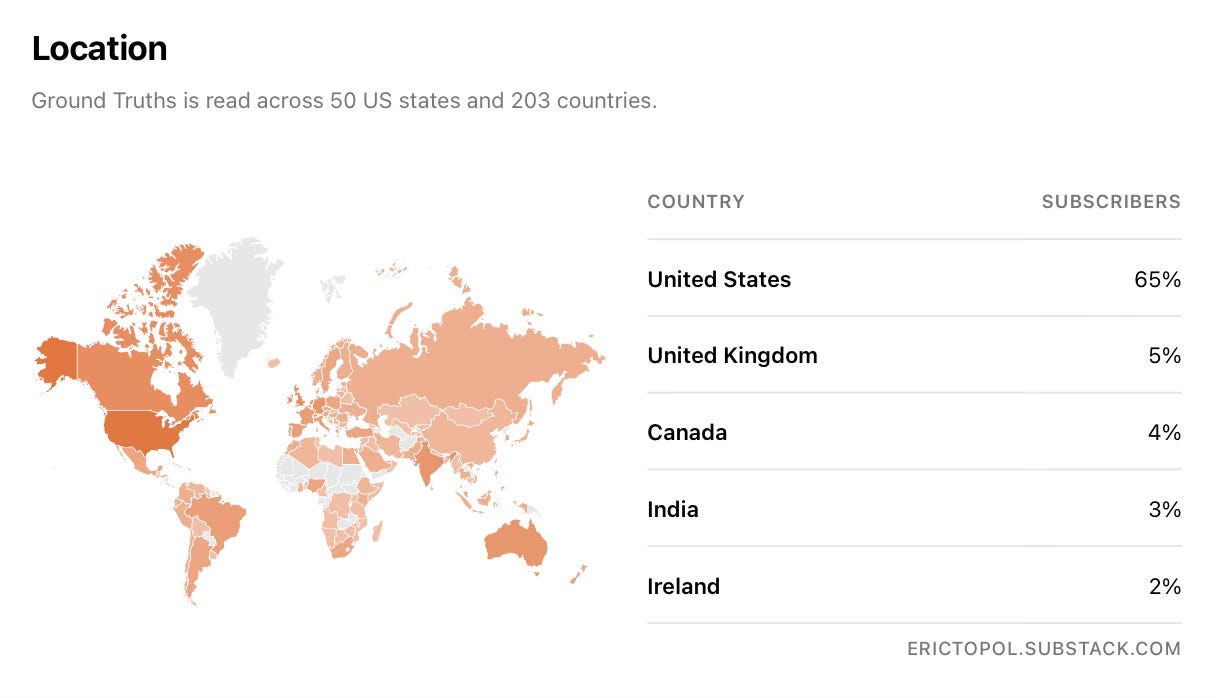
Ground Truths now has subscribers in 203 countries!













Share this post