Superimposed on an impressive body of work on the blood-brain-barrier and immune system, Prof Akassoglou and her collaborators just published an elegant study in Nature that centered on the direct binding os the SARS-CoV-2 spike protein to fibrin with marked downstream pro-inflammatory effects. The findings and potential treatments have implications beyond Covid, Long Covid to other neurologic diseases.
Full videos of all Ground Truths podcasts can be seen on YouTube here. The audios are also available on Apple and Spotify.
Transcript with links to audio and to relevant papers, graphics
Eric Topol (00:07):
Well, hello this is Eric Topol with Ground Truths, and with me today is Katerina Akassoglou. She is at the Gladstone Institute and she is a remarkable neuroimmunologist who has been doing extraordinary work for three decades to unravel the interactions between the brain, blood vessels and the role of inflammation. So Katerina, there's a lot to discuss, so welcome.
Katerina Akassoglou (00:40):
Thank you. Thank you so much. It's a great pleasure to join.
By Way of Background
Eric Topol (00:43):
It's really interesting going back in your career. First of all, we're thankful that you immigrated here from Greece, and you have become one of the leading scientists in this discipline of important discipline of neuroimmunology, which is not just about Covid that we're going to talk about, but Alzheimer's and neurodegenerative diseases. This is a really big hot area and you're definitely one of the leaders. And what I was impressed is that all these years that you've been working on the integrity of the blood-brain barrier, the importance of fibrinogen and fibrin, and then comes along the Covid story. So maybe what we can do is start with that, which is you've made your mark in understanding this whole interaction between what can get into the brain, through the blood-brain barrier and incite inflammation. So this has been something that you've really taken to the extreme knowledge base. So maybe we can start with your work there before we get into the important seminal Nature paper that you recently published.
Katerina Akassoglou (01:57):
Yes, of course. So since very early on, I was still a graduate student when we made the first discovery and at the time was like mid-90s, so it was really ahead of its time. That dysregulation of cytokine expression in the brain of mice was sufficient to induce the whole cascade of events, triggering neurodegeneration, demyelination in pathological alterations, very reminiscent of multiple sclerosis pathology. And it was really hard to publish that study at the time because it was not yet accepted that this regulation of the immune system modeling the brain can be linked to neurodegeneration. So that was 1995 when we made that discovery, and I became really interested, what are the pathogenic triggers that actually polarized the immune cells in the brain? So with this, of course, this transgenic animal was expressing TNF, it was an artificially made animal that we made, but naturally what were the triggers that would polarize the innate immune cells? So I looked really early on in this mice and what I found was that the very first event was leaks of blood-brain barrier. It was opening of the blood-brain barrier in this mouse before inflammation, before demyelination, before neuronal loss. And this is really what shaped the question that, is it possible that these blood leaks that happened very early in the pathology, could this be the instigators of pathogenic inflammation in the brain?
Eric Topol (03:34):
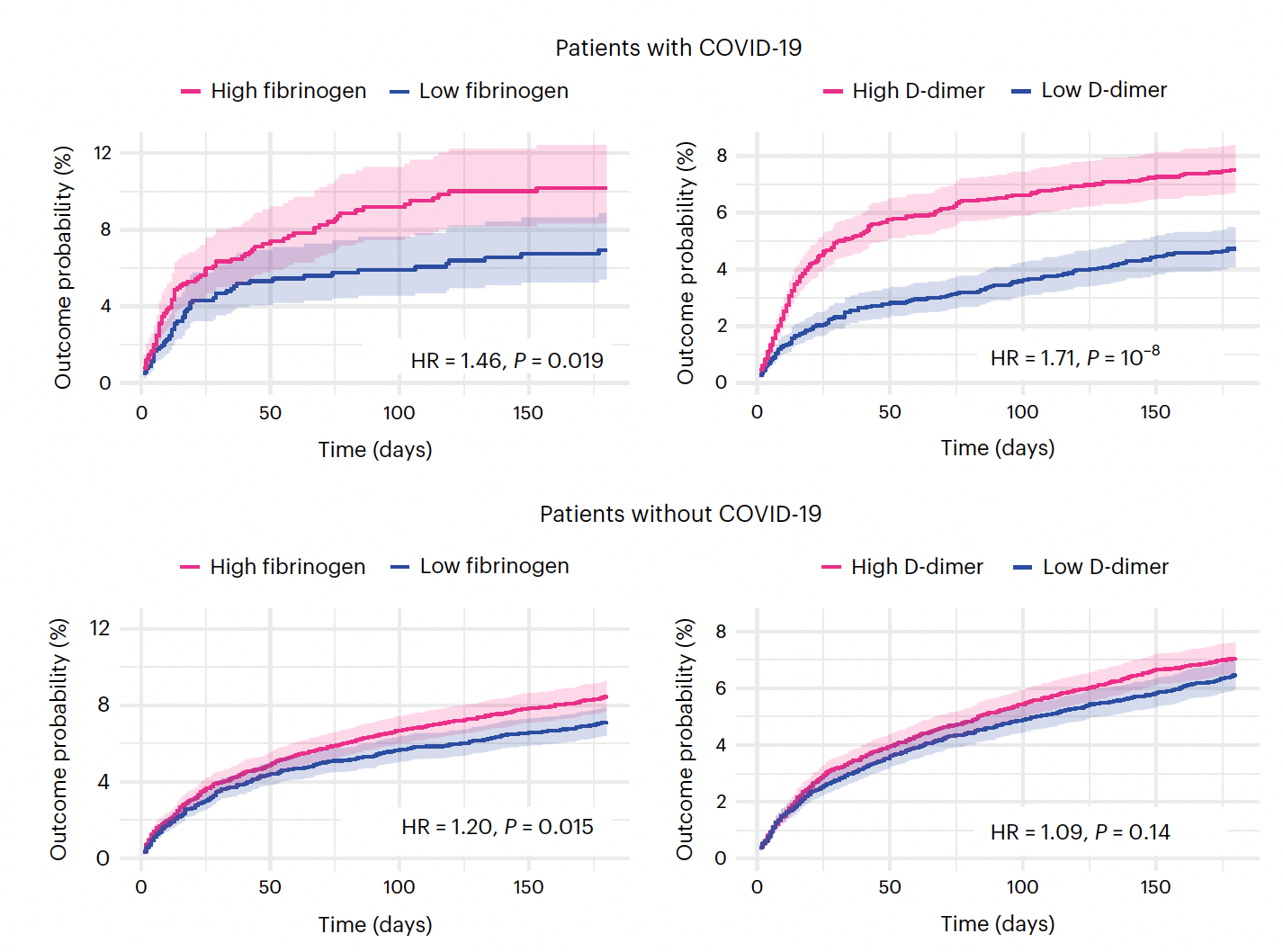
Yeah. So in a way, you got at this question because of the chicken-and-egg and what happens first, and you got to the temporal saying, which happened first as you said, the leak before you could see evidence of inflammation and being able to study this of course in the experimental model, which you couldn't really do in people. And what I love about the description of your career, which has been quite extraordinary contributions is connecting the dots between the blood, the inflammatory response and the brain. Perhaps no one has done that like you have. And before we get into the recent paper, a lot of people are not aware that a year ago, a group in the UK known as PHOSP-COVID, they published a really important paper in Nature Medicine of over 1,800 people who were hospitalized with Covid and they found that fibrinogen was the best marker for cognitive deficits at 6 and 12 months (Figure below)
(04:40):
So that's just one of many papers, but it's a particularly well done study that already before you got into this work that recently published had emphasized fibrinogen. And by the way, again, having spent a lot of years in clots in the arteries, for me, we have to just get it down to fibrinogen plus thrombin gets you to fibrin. Okay, so fibrin is a major player here when fibrinogen is cleaved. So here we have the basis that you established, which is the fibrinogen leakage into the brain, activating inflammation, activating microglia, which like the macrophages of the brain and inciting the whole process. And before we close, I want to not just talk about Covid, but Alzheimer's too. But now let's get into the study that you did, [Fibrin drives thromboinflammation and neuropathology in COVID-19] which is striking, I mean really striking. And can you kind of take us through, because you not only demonstrated the importance of fibrin in inciting neuroinflammation in this model, but also how you could reverse it or prevent it. So this, and you looked at it in many different ways, this was a systematic approach. Maybe you can take us through how you were able to make such compelling evidence.
The Multimodal Evidence
Katerina Akassoglou (06:09):
Yes, thank you. First of all, thank you for bringing up the human relevance because this was also our inspiration for the work that we did in the Covid study. So as you mentioned in Covid patients, fibrinogen unbiased mass spec analysis was identified as the predictive biomarker for cognitive impairment in Long Covid patients. And this was in addition to also neuropathology data about the abundance of fibrin deposition in the brain. And these were studies that were done by NIH that have found deposition of fibrin in the brain and the reports for the abnormal and puzzling coagulation in Covid that is not setting other infections and also in many cases not always relating with the severity of symptoms. So even mild cases of Covid also had increased coagulation. I was really intrigued by this human, all this evidence in human data, and I thought that maybe the way that we're thinking about this, that it's systemic inflammation that drives the clotting.
(07:24):
Maybe there's another aspect to this. Maybe there is a direct effect of the virus with the coagulation cascade, and in this way maybe this can be an instigator of inflammation. So this was the original idea to be able to reconcile this data from the clinic about why do we have this prevalence of coagulopathy in Covid. And of course, the second question is, could this also be a driver of the disease? And of course, we're in a unique position because we have been studying this pathway now for over 20 years to have all the toolbox, the genetic toolbox, the pharmacologic toolbox to be able to actually really address these questions with genetic loss of function studies, with a blood innate immunity multiomics pipeline that we have set up in the lab. And of course, with preclinical pharmacology in our ABSL3 facility. So we had the infrastructure in place and the source in place to actually really dissect this question with both genetic tools as well as also technology platforms.
Eric Topol (08:29):
And you had in vivo imaging, you're the director of in vivo imaging for Gladstone and UCSF. So you do have the tools to do this.
Katerina Akassoglou (08:38):
Yes. The imaging that you mentioned is really important because this is, we employed that very early in our studies over now 15 years ago. And the reason was sometimes from snapshots of histopathology, you cannot really understand the sequence of events. So by being able to image these processes, both neuronal activity, microglia activation, infiltration of peripheral cells in the brain, this is how we could see the steps that what happens early on and to be able to answer these chicken-and-egg questions that you mentioned. So these were very, they're very important experiments, especially at the beginning because they were hypothesis driving and we were able to ask the right questions to drive our research program.
Eric Topol (09:26):
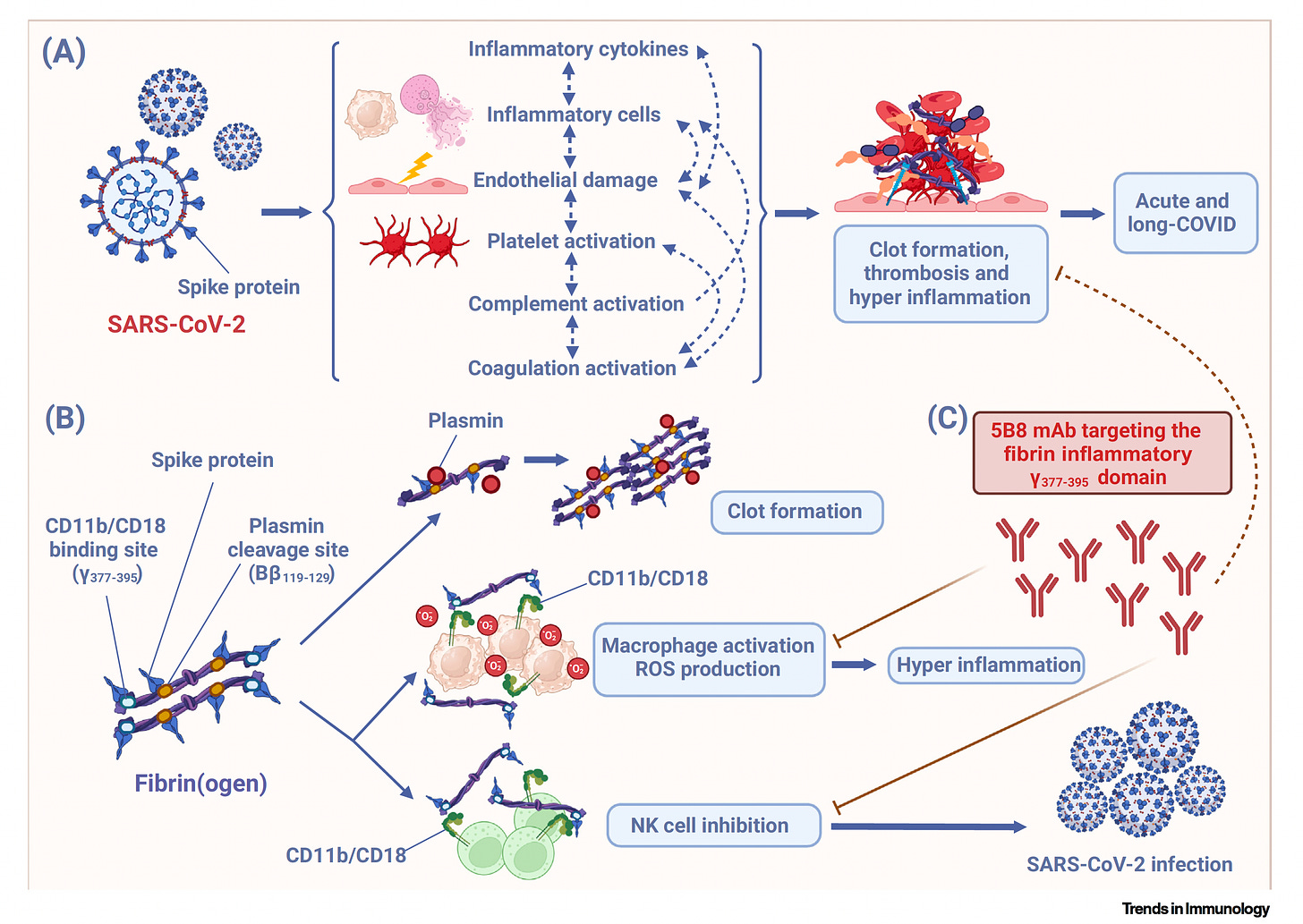
Now was the binding of the spike protein to one key site in fibrinogen, was that known before? [See outstanding Figure below from Trends in Immunology]
Katerina Akassoglou (09:36):
No, this was not known. So there was evidence that there are abnormal clots in Covid, but it was not known whether the spike protein would directly bind to protein to the coagulation cascade. So one of the key discoveries in our study was to use peptide array mapping and be able to identify not only the binding, but exactly the domains on fibrin that spike binds too. And what we found was two key domains, one the inflammatory domain and the other the plasmin binding site, which is important for fibrin degradation. So this suggested a potential dual deleterious role for this interaction, both by maybe affecting inflammation, but also delaying fibrinolysis, which is the degradation of this toxic protein from the brain. And indeed, we found that this interaction was responsible for all these two aspects, including decreased degradation, more inflammation, but also at the same time increased, increased coagulation. So it was a really pathogenic interaction.
Eric Topol (10:47):
Yeah, actually it's pretty striking. You have these two sites, the plasmin cleavage site of fibrinogen, which as you say, we knew there was a problem with clots. We knew that, but we didn't know exactly the spike protein how exactly it was implicated, particularly with fibrinogen. And then this other site, the CD11b-C18, now that's fancy for surface receptors of macrophages. And basically, this is critical because it's this microglia activation in the brain, and I know you saw it in the lungs as well through this other site that spike protein activated. So you had a twofer here of things that you discovered that the SARS-CoV-2 spike protein was capable of doing. This was a really big revelation. And then you also looked at mice that were genetically manipulated. So maybe you can, because before we get to your antibody monoclonal, the ways that you proved this were, I mean, one thing after another is really systematic. So maybe you can teach us about that.
Establishing Causality
Katerina Akassoglou (12:08):
Yeah, sure. So the first was about chemistry experiment. So this of course, we had to get to the next step to see is there any causality for this pathway. So we employed genetic loss of function studies and we had knockout mice, either fibrinogen knockout mice, this mice have all blood proteins except fibrinogen, and they have a delay in coagulation so they don't clot properly. But we also had a mutant mouse, which is a fibrinogen NK mouse. And this was a mutation only within this inflammatory domain that you mentioned, inflammatory domain that binds to C11b-C18. Other names for this is of course complement receptor 3, Mac-1 (αMβ2). It's the same, many names for this receptor, that as you mentioned, is expressed not only in microglial in the brain, but also peripheral immune cells including macrophages as well as also neutrophils which are CD11b expressing.
(13:12):
So we now have genetic models to be able to look at both complete depletion of fibrinogen, but also a very specific mutation and very selective mutation that only blocks the inflammatory properties without affecting the properties of fibrin in hemostasis. And these mice were made many years ago by a very close collaborator, Jay Degen at the University of Cincinnati. So what we found is that when we block either the inflammatory domain or we completely deplete fibrinogen, there was this profound protection after infection in internasal infection with the virus in lung inflammation. And this was both suppression of oxidative stress and this pathogenic inflammation in the lung, but also decreasing fibrosis, which has been associated with also Long Covid. And the surprise came from the transcriptomic data. So when we did transcriptomic analysis in this mice in the lungs, we found perhaps the expected decrease in the immune signatures in macrophages. This was in line with our previous work in, as you mentioned, Alzheimer's models, multiple sclerosis models. But what also was really surprising is there was that genes that are associated with activation of NK cells were upregulated. And of course this was the first time we had infected these mice, previously we had not done an infection before. So I think that maybe because of this region we had not seen before in our data this immunomodulatory role of fibrin that not only surprises the macrophage response, but also increases these NK cells that are important for viral clearance.
Eric Topol (15:00):
So again, the finding another important unique finding is the natural killer (NK) cells and effect there from the activation of this, as you said, the inflammation site or the CD11b-C18 that we've been talking about. So now another layer of this, a dimension of your Nature paper was that you tested an antibody that you already had developed so-called 5B8. A monoclonal that specifically binds to the domain of the one we're talking about this inflammation domain of fibrinogen. So can you tell us about what that showed?
Katerina Akassoglou (15:45):
Yes, so we tested this antibody in different models of Covid, which were both models with neuroinvasion and models without neuroinvasion. So we used both transgenic mice for hACE2, the human ACE2 infected with Delta, but we also use mouse adapted viruses like Beta that is just in the wild type mice with no transgenic being involved that these are without neuroinvasion. And we wanted to see if the antibody had any potential protective effects. And what we found is that the antibody protected from inflammation in the lung. So the data looked so similar with a genetic mutation of this pathway, protection from inflammation, decreased fibrosis, increased viral clearance, so decreased spike and viral proteins in the lungs. But we also found a protection in the brain. So the brains of this mice, including both the models we used with neuroinvasion and without, they both have had microglia activation in the brain. And we also found neuronal loss in the Delta infected mice and the antibody protected from both neuroinflammation but also improved neuronal survival in the mice. Showing that there can be this despite regardless of which model we used, there was this protective effect suggesting that by blocking fibrin, either the periphery or in the brain, this could be protected for these models.
Eric Topol (17:28):
Yeah, so I mean this is fascinating because until now, until this report of yours and your colleagues at Gladstone, there was knowledge that there would be neuroinflammation from Covid, both in patients from various biomarkers and imaging as well as in experimental model. But what this did was take it to the fibrin story, and I guess that's one of the questions you nailed that how important fibrin is, but that doesn't necessarily rule out other triggers of neuroinflammation, right?
Katerina Akassoglou (18:04):
Oh, absolutely not. So I think that this is one of the mechanisms that can be very important, especially in some patients. But we know that there are additional of course mechanisms of neuroinflammation including auto-antibody responses, as well as also endotheliopathy that are persistent endotheliopathy, this can be interacting also with each other. So I think that it's important for future research that we understand how do these mechanisms feed into each other? Are there a positive feedback loops between autoimmune mechanisms and coagulopathy and endothelial dysfunction with inflammation? But I think most importantly, I think that if we're thinking of this in the context of patients, can we identify patients with mechanism that might be more prevalent in specific cases of Long Covid and tailor our potential future clinical trials towards the needs of Long Covid patients?
Towards Treatment
Eric Topol (19:06):
Absolutely. I did interview some months back on Grounds Truths, Michelle Monje at Stanford, who I'm sure and interact with, and she's also works not so much on the fibrin side, but on neuroinflammation and the likeness between this condition in people and chemo brain because of the inflammation that's seen there. So we've talked about the multiple triggers that could contribute to brain inflammation, which I think most people would say in Long Covid this is one of the most, besides obviously the lack of energy, the profound fatigue and disability, but the cognitive function hit, not just brain fog is often profound. And we've just seen some reports about that, and particularly in hospitalized patients, how bad that can be. So that gets us to a potential treatment. Now, one of the things that's out there dangling, there's many things that people have talked about in terms of why can't we have a treatment for Long Covid?
(20:13):
And now of course this fibrin pathway, if you will, lends itself to many possibilities, whether it's anticoagulants or fibrinolytics like a tPA or things like nattokinase, which is a Japanese food enzyme that you could get at the nutrition centers or whatever. What are your thoughts? Because we don't have any good studies. There are all these little, tiny studies and they don't provide much conclusion, and you have an antibody that could potentially be effective. As I understand it, you set up a company some years ago, Therini Bio and used to be called MedaRed. You're the first woman scientist at Gladstone to develop a spin out company, which is another point of congratulations on that. But could the antibody be tested in patients or what do you think about these other possibilities?
Katerina Akassoglou (21:15):
Yes, yes. These are great questions. So first of all, the different approaches that you mentioned have very different mechanism of action. So degrading fibrin, the degradation products of fibrin also can have deleterious effects. The dimer, for example, can be very pro-inflammatory. So at the same time, blocking coagulation can also have a diverse effects because this can lead to excessive hemorrhage. So the approach that we took was to selectively block the inflammatory properties of fibrin without affecting beneficial effects of the molecule in normal hemostasis. So the challenge when I made the antibody was to be able to dissect these two functions of fibrin. It's our most important clotting factor, but at the same time, a molecule with profound pro-inflammatory capacity. So the observation that these two domains, the clotting domain and inflammatory domain were not overlapping, was really the foundation of this invention was that we could maybe create this antibody to be able to target them in a selective way.
Other Neurologic Conditions
(22:31):
So the antibody I developed is neutralizing blood toxicity by blocking the inflammatory domain of fibrin without adverse coagulation effects. And it's now completing phase one trials. So it has already completed the single ascending dose at 40 milligram per kilogram. It's interim data were announced already for this trial, with no safety signals. So if the antibody completes this year, the phase one trials, then it should be possible to be tested in different patient populations. You mentioned before chemo brain, and I think it's important that we think that blood-brain barrier disruption occurs among many neurological conditions, and it's an early event associated with early disease onset and worse prognosis in multiple sclerosis, Alzheimer's disease, traumatic injuries. So I think that it's by developing a strategy, therapeutic strategy to neutralize blood toxicity, this can have applications in a wide range of neurological conditions with vascular dysfunction.
Eric Topol (23:54):
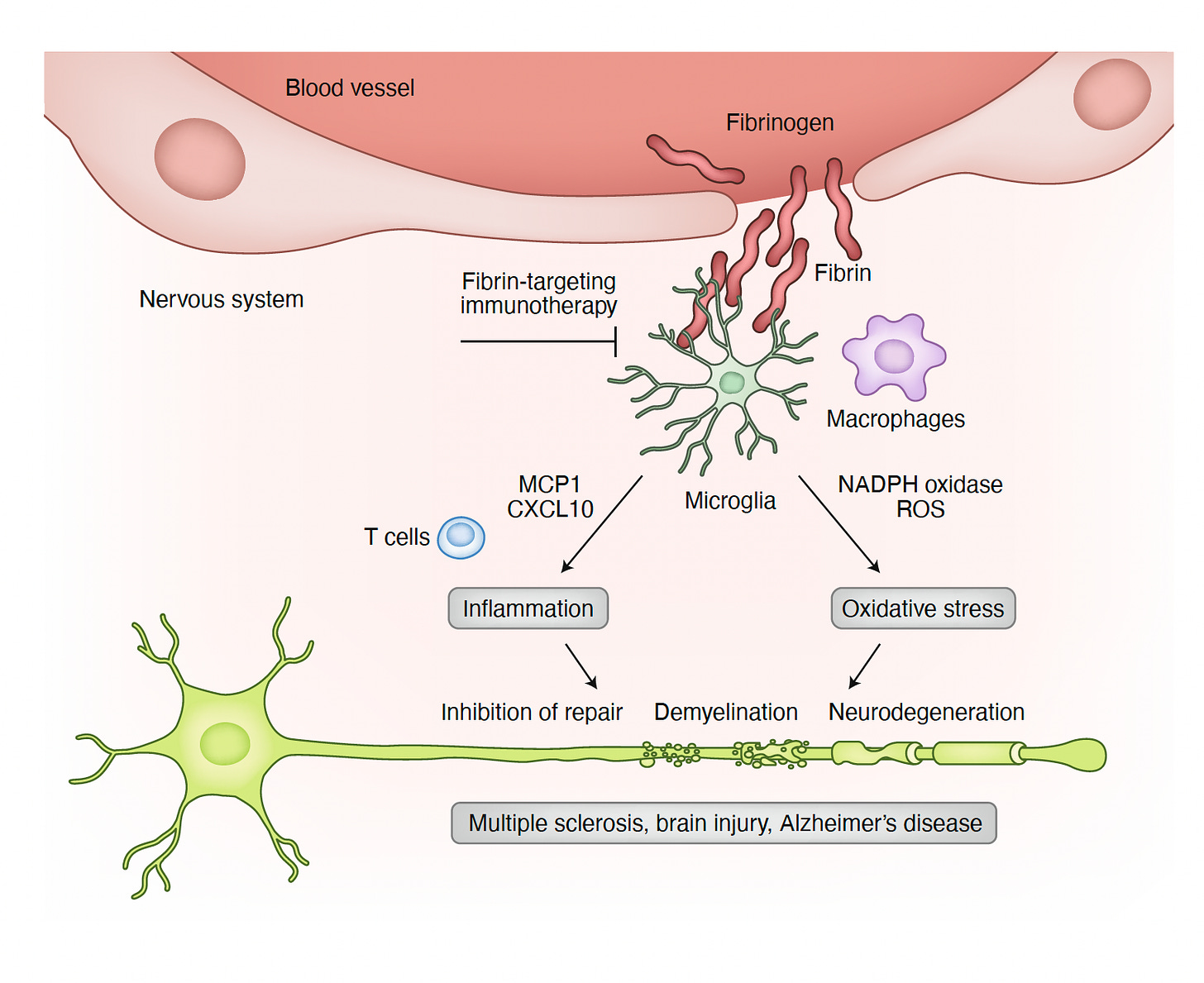
Yeah, no. In your Nature Immunology 2020 piece [Figure below], you started with the 1883 identification of multiple sclerosis (MS) lesions were “engorged with blood”, the first link between blood leaks and brain inflammation. So this has enormous potential. And what I like about this Katerina is that you've dissected the clot component versus the inflammatory trigger of the fibrinogen and fibrin story. And this is so vital because if you keep throwing these things that just going to work on the clot and not deal with the pro-inflammatory consequences, then you're going to get the wrong impression that clots are not that important. And by the way, you did mention, and I want to come back to that too, endothelial inflammation, which is another feature of Long Covid is another kind of interactive part of this because when the lining of the blood vessel is inflamed, it will attract microthrombi and also be a participant in this whole affair. What do you think about Alzheimer's and the prospects of being able to interfere with Alzheimer's? We have 20 years in someone before this process takes hold and meets clinical manifestations. Would an antibody like this ever be useful along the way?
Katerina Akassoglou (25:29):
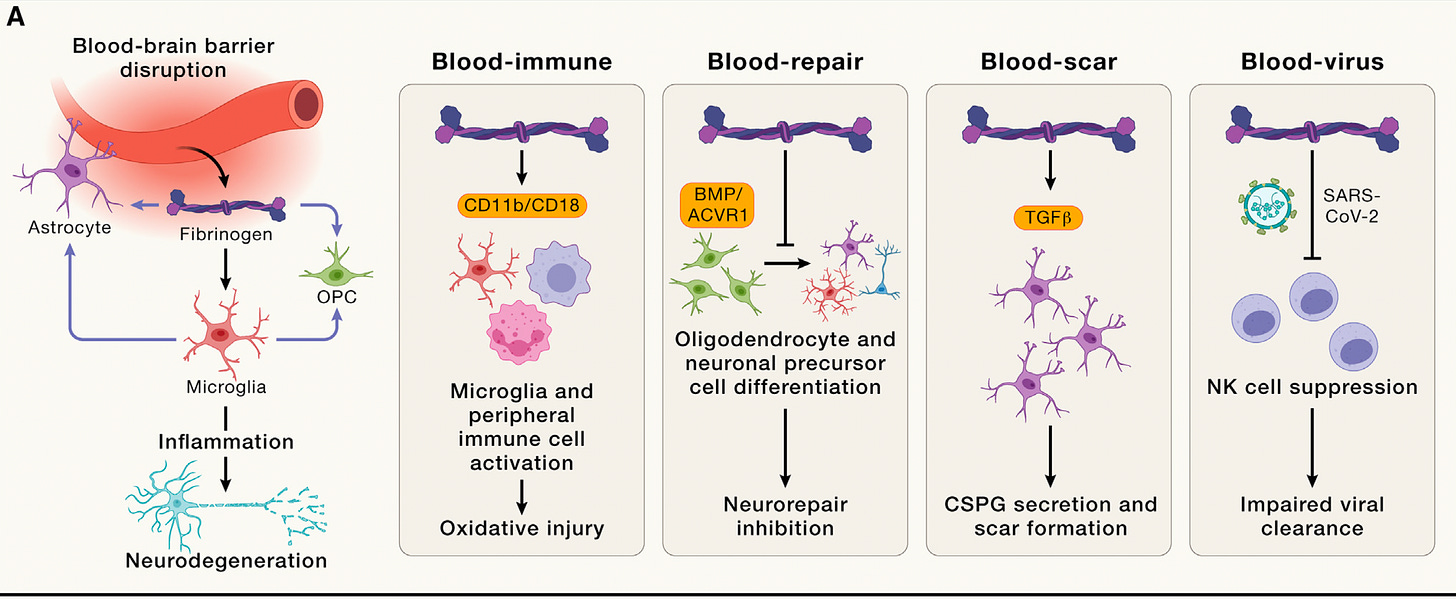
Yeah, so well, our antibody was tested first in Alzheimer's, this models when it was originally published, and we performed reversal trials in Alzheimer's models. So we dosed mice when they have established amyloid plaques, microglia activation, neuronal loss, and we could reverse this effect so it could increase cholinergic neurons in mice, reduce inflammation in a very selective way, only the neurotoxic part of inflammation and for genetic depletion of this pathway with akin mice in Alzheimer's disease. Also, improves from cognitive impairment, and we now have a new paper in Cell Press that is showing this effects also with really nice and unbiased machine learning models for behavioral segmentation [Figure below].
So I think that there is the data both from genetic studies and the antibody show projection in Alzheimer's disease. And of course, as you might have read the recent Lancet report from the Lancet committee on dementia that identified the vascular risk factors as the key contributors, especially post sporadic cases of Alzheimer's disease that is over 90% of Alzheimer's disease that is not genetically linked.
(26:58):
So I think that there is a real need in Alzheimer's disease to be able to block this vascular induced pathology. And an antibody like the fibrin neutralizing therapy could be positioned to be protective from the vascular induced immune-mediated neurodegeneration in this disease as well. I mean, ultimately, I think that we need to be thinking the terms of efficacy. So we want to have a drug that is efficacious, but we also want it to be selective. And the selectivity is really important because the immune system has so many protective functions. So if we block phagocytosis, we end up with more debris, decrease of neurorepair, anti-myelination. So by blocking a ligand here and not blocking, not eliminating a cell type or blocking a global pathway in this cell, but biologic a single ligand, I think we have been able to achieve this balance between efficacy, but also safety because we only block this neurotoxic populations and not the entire innate immune response that also has been beneficial for metastatic functions in the brain.
Blocking Neuroinflammation
Eric Topol (28:19):
So you're bringing up another critical concept about targeting the inflammation, this kind of goldilocks story of how much you interfere with the immune response and how much you are able to reduce the adverse pro-inflammatory effects. So that gets me to what if we don't know in any given patient how much fibrin is having a role in their Long Covid. Although we know it has to be a prominent feature because we saw it in, not just a hospitalized patient series that I mentioned we reviewed, but other papers as well. But what about if you just try to take on inflammation like through a GLP-1 drug or cGAS–STING or any of these really strong anti-inflammatory pathways. Do you see a difference in a generalized approach versus a specific approach that is really fibrin centered?
Katerina Akassoglou (29:22):
Yeah, so we have a focus actually on both because we wanted to dissect the downstream intracellular pathways of fibrin, and it's interesting that we can find specific inflammatory mediators that potentially can also be targeted as well, to be able to preserve that specificity, which I think is really important because if we don't preserve the specificity, we'll end up with a lot of adverse effects by eliminating major immune responses. But the point that you raised I think is really important because it's not enough to have an efficacious and selective drug if you don't know the patient population that will benefit from this drug. So I think that in addition to the drug discovery studies, it's important to develop also biomarker programs with both fluid biomarkers, but also imaging biomarkers to be able to identify the patient populations that will benefit from such treatment.
(30:25):
So if for example, a patient population has a fibrin deposition, blocking only downstream might not be enough, and it might be really important to neutralize this fibrin toxicity in the brain of patients. And with our target engagement studies, we show that at least in animal models, the antibody can be there. So I'm very encouraged by also programs that are going on now in the scientific community to develop noninvasive ligands to be able to image fibrin in the brain that are already tested in different patient populations like multiple sclerosis. Because I think we're going to learn so much from the biology as we start interrogating and asking these questions now in different patient populations.
Eric Topol (31:14):
I think that's a vital point you're making because the success of a clinical trial here in a clinical syndrome that is mosaic with lots of different types of pathways. If you can nail down the patients that would have the most to stand to benefit from a particular intervention, that the chance of you not missing the benefit that is matching the marker, what image marker or other markers is so vital. Well, we've talked, I think, about some fascinating discoveries that you and your colleagues have made. I mean, it's really extraordinary, and obviously we need this in Long Covid. But you know what, Katerina, it's almost made me think that you were warming up to this for three decades, that somehow or other you were working on all this stuff and then came Covid. Is that how you see it, that somehow or other you didn't know that all the work you were doing was going to wind up in this space?
Katerina Akassoglou (32:18):
Oh, I never thought I would work in a virology project. This collaboration started over Zoom with Warner Greene. We were both sheltering in place. It was the beginning of the pandemic, and the first reports were coming out about this puzzling coagulopathy. And our labs were hardly operational at the time, as you know, we had to close down our labs for a while. And however, this was a very big problem, and we thought that this is our role as scientists. If we feel that we can contribute and we have the tools to contribute, we felt that it's important that we pivot some part of our research, and even we wouldn't be doing this before, but it was important to pivot a part of our research and collaborate. And I think studies like this, this study would have been impossible without a team of collaborators. As you know, there were over 50 scientists involved at Gladstone, UCSF, UCLA, UCSD, Stanford University. Without collaboration, this study wouldn't be possible. So I'm really grateful to everyone who came together to solve this problem because I think that's what scientists should be doing. We should be solving problems as they arise.
Eric Topol (33:41):
Well, and also, I think a lot of people don't realize that, for example, when the Covid vaccines came along, people think, oh, well, it all got done in 10 months since the sequence of the virus, when in fact it took 30 years at least between all the factors that went into having an mRNA and sequencing virus and nanoparticles. And in many ways, your arc of this work is like that because it took three decades to have all the tools and the basic understanding, the antibody that you had developed for different reasons and this fascinating unraveling of what's going on in the model and undoubtedly in some patients at least as well. So before we wrap up, have I missed anything about this just remarkable work you've done?
Katerina Akassoglou (34:33):
Oh, thank you. I just want to thank you for this discussion and thank you for emphasizing the different areas and the different decisions that this pathway can have implications both for our understanding, our basic understanding of the blood brain immune interface, as well as also potential translation. And I think that the curiosity sometimes of how things work, I never thought it would work on Covid, like you mentioned at the beginning, but I think that basic science and curiosity driven science can sometimes lead to discoveries with translational implications that hopefully might benefit patients one day.
Eric Topol (35:21):
Yeah, well, undoubtedly it will. We're indebted to you, Katerina and all the folks that you have teamed up with, connecting the dots at the neurovascular interface. Phenomenal work and will follow the subsequent with great interest and it will likely not just a story about Long Covid, but other areas as well, so thank you.
*********************************
Thanks for listening, reading or watching!
The Ground Truths newsletters and podcasts are all free, open-access, without ads.
Please share this post/podcast with your friends and network if you found it informative!
Voluntary paid subscriptions all go to support Scripps Research. Many thanks for that—they greatly help fund our summer internship programs.
Thanks to my producer Jessica Nguyen and Sinjun Balabanoff for audio and video support at Scripps Research.
Note: you can select preferences to receive emails about newsletters, podcasts, or all I don’t want to bother you with an email for content that you’re not interested in.











Share this post